Comments on the NBPP proposed rule for 2025

Administrator
Centers for Medicare & Medicaid Services
U.S. Department of Health and Human Services
200 Independence Avenue, SW
Washington, DC 20201
RE: Comments on Notice of Benefits and Payment Parameters for 2025 Proposed Rule [CMS-9895-P]
Dear Administrator Brooks-LaSure:
The HIV+Hepatitis Policy Institute, a leading national HIV and hepatitis policy organization promoting quality and affordable healthcare for people living with or at risk of HIV, hepatitis, and other serious and chronic health conditions, is pleased to offer comments on the Notice of Benefits and Payment Parameters for 2025 proposed rule. Earlier we submitted comments on the 2025 Draft Letter to Issuers in the Federal-facilitated Exchange, which can be found here. In those comments, we focus on adverse tiering in drug formularies and the lack of enforcement by CCIIO and state regulators in ensuring issuers are in compliance with ACA nondiscrimination and other patient protections.
We appreciate all you are doing to make healthcare more accessible and affordable for beneficiaries, including several proposals contained in the proposed rule. While we support several of them, this letter focuses on those issues that impact access and affordability of prescription drugs.
- Now that the District Court for D.C. in HIV and Hepatitis Policy Institute et al. v. HHS et al. has struck down the section of the 2021 Notice of Benefits and Payment Parameters rule that allowed issuers to decide if copay assistance can count or not, and that same Court has clarified, at the government’s request, that the 2020 Notice of Benefits and Payment Parameters rule is now in effect, issuers must count copay assistance in most instances and not implement copay accumulators. We urge CMS to issue guidance reminding plans that the 2020 NBPP rule is now in effect and must be followed. While CMS has stated it will issue new rules on this issue, if they do, it must ensure that copay assistance must count as cost-sharing. The federal government should also drop its appeal of the lower court’s decision.
- We are pleased CMS is proposing to codify existing policy that prescription drugs covered in excess of the state’s benchmark plan are considered essential health benefits (EHB) and are therefore subject to EHB protections, including annual cost-sharing limits. In response to CMS’ request, we provide examples of the use of these schemes in the private insurance market and urge the administration to clarify that the regulation applies to all plans.
- We are pleased that CMS is continuing to require standardized plans in an effort to increase patient healthcare affordability, including prescription drugs, which for the most part requires the use of reasonable copays. Some of the copay amounts, particularly on the specialty tier, still need to be lowered for patients. Additionally, for many drug tiers, beneficiaries are still required to meet a high deductible before taking advantage of the capped copays. We support CMS’ proposal for 2025 that reduces the number of standard plans, but with an exceptions process.
- We voice strong support for adding patient representatives to Pharmacy and Therapeutics Committees beginning in 2026.
- We support CMS’ proposal to move away from U.S. Pharmacopeia (USP) Medicare Model Guidelines to USP Drug Classification System to develop the essential health benefit component for prescription drugs.
Copay Assistance & Definition of Cost-sharing
Now that the District Court for D.C. in HIV and Hepatitis Policy Institute et al. v. HHS et al. has struck down the section of the 2021 Notice of Benefits and Payment Parameters rule that allowed issuers to decide if copay assistance can count or not, and that same Court has clarified, at the government’s request, that the 2020 Notice of Benefits and Payment Parameters rule is now in effect, issuers must count copay assistance in most instances and not implement copay accumulators.
We urge CMS and other agencies to issue guidance reminding plans that the 2020 NBPP rule is now in effect and must be followed. While CMS has stated it will issue new rules on this issue, if they do, it must ensure that copay assistance must count as cost-sharing. The federal government should also drop its appeal of the lower court’s decision.
The issue of copay assistance has been debated for several years and we have been extremely disappointed in the government’s stance against patients and in defense of the insurers and PBMs.
Now that there is no doubt that the 2020 NBPP is now in effect, it must be followed. That rule states:
“Notwithstanding any other provision of this section, and to the extent consistent with state law, amounts paid toward cost sharing using any form of direct support offered by drug manufacturers to enrollees to reduce or eliminate immediate out-of-pocket costs for specific prescription brand drugs that have an available and medically appropriate generic equivalent are not required to be counted toward the annual limitation on cost sharing (as defined in paragraph (a) of this section). 45 C.F.R. § 156.130(h)(1)”
As HHS explained in adopting the rule: “Where there is no generic equivalent available or medically appropriate,” manufacturer assistance “must be counted toward the annual limitation on cost sharing.” 84 Fed. Reg. at 17,545
Now that this has been settled, and since there has been so much confusion on what is in effect, it would be beneficial for the federal government to issue tri-agency guidance to alert issuers that this is what is in effect. Additionally, all regulators must enforce the requirements. The U.S. government cannot merely disregard the District Court ruling and allow insurers to do what they have been doing prior to the Court ruling.
We also trust that the District Court ruling puts to rest some of the misinformation that issuers and PBMs have perpetuated that the government has echoed in their statements and briefs to the court. Based on all the briefs submitted in the case, the Court concluded what patient groups have long been stating: copay accumulators increase patient costs, increase drug manufacturer payments, increase insurer revenues, and are not drug discounts.
There is no doubt that patients need copay assistance in order to afford their prescription drugs, due to high cost-sharing, often expressed in term of co-insurance based on the list price of a drug, along with high deductibles.
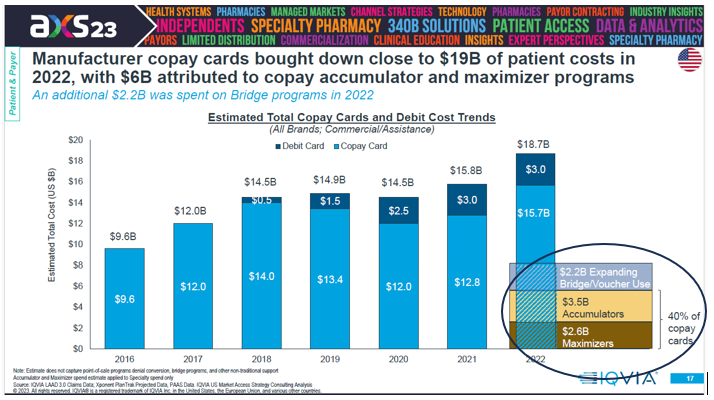
In 2022, according to IQVIA, patient out-of-pocket costs in 2022 were $82 billion for prescription drugs. That was an increase of $3 billion from 2021.[1] Manufacture copay assistance brought down patient costs by nearly $19 billion and accounted for 23 percent of the out-of-pocket costs. Over the last five years, it accounted for $80 billion.[2]
Without these contributions, the American people would have had to come up with all this money, which most people do not have.
Consider the following:
- According to the Commonwealth Fund 2023 Health Care Affordability Survey:
- Large shares of insured working-age adults surveyed said it was somewhat or very difficult to afford their health care: 43 percent of those with employer coverage and 57 percent with Marketplace or individual-market plans.
- Many insured adults said they or a family member had delayed or skipped needed health care or prescription drugs because they couldn’t afford it in the past 12 months: 29 percent of those with employer coverage and 37 percent covered by Marketplace or individual-market plans.
- Cost-driven delays in getting care or in missed care made people sicker. Fifty-four percent of people with employer coverage who reported delaying or forgoing care because of costs said a health problem of theirs or a family member got worse because of it, as did 61 percent in Marketplace or individual-market plans.
- Insurance coverage didn’t prevent people from incurring medical debt. Thirty percent of adults with employer coverage were paying off debt from medical or dental care, as were 33 percent of those in Marketplace or individual-market plans.
- Medical debt is leading many people to delay or avoid getting care or filling prescriptions: more than one-third (34 percent) of people with medical debt in employer plans while 39 percent in Marketplace or individual-market plans.[3]
- According to CMS, the 2024 silver plan median deductible increased from $5,440 in 2023 to $5,726 in 2024, an increase of 5 percent from 2023 and 19 percent from 2020. The bronze plan median deductible in 2024 is $7,239, which is a decrease of 3 percent from 2023, but an increase of 6 percent from 2020.[4]
- According to CMS’ 2022 National Health Expenditures report, while overall healthcare spending grew at 4.1 percent in 2022, out-of-pocket spending increased substantially higher at 6.6 percent in 2022 to $471.4 billion. For prescription drugs, out-of-pocket spending totaled $56.7 billion, or 14 percent of the total spending on prescription drugs. This represents an increase of 11.6 percent in 2022 after slower growth of 6.4 percent in 2021.
However, for hospital care, which accounts for more than three times more of the total spending than prescription drugs, patients were responsible for paying only 2.6 percent. Despite the much smaller total amount of spending for prescription drugs, the out-of-pocket spending for prescription drugs ($56.7 billion) was higher than all the out-of-pocket spending for hospitals ($35.1 billion).[5] - According to an IQVIA analysis, due in part to high costs, an estimated 92 million prescriptions were abandoned at the pharmacy in 2022 (this compares to 81 million in 2021), with the abandonment rate over one in three for prescriptions above $75 in out-of-pocket costs. Additionally, for prescriptions with a final cost above $250, 53 percent are not picked up by patients, as compared with 7 percent of patients who do not fill when the cost is less than $10.[6]
While after premiums are paid there are cost-sharing limits, they too are rising. For plan year 2025, CMS has set the maximum out-of-pocket responsibility at $9,450 for an individual and $18,900 for all others. Due to the proliferation of high deductible plans, depending on the drug, a patient may be required to pay the total amount of $9,500 all at once for their medication at the beginning of the year.
While there has been much attention to the list price of medications, out-of-pocket costs are set by the insurers. Due to insurance benefit design, they are forcing beneficiaries to pay a high amount of costs, especially compared to other healthcare services. Since patients cannot afford these costs, copay assistance is essential.
While it is not necessary for CMS to issue new regulations regarding cost-sharing since the 2020 rule is sufficient, if it does, it is critical that copay assistance be included as part of cost-sharing. If it were not, we believe some manufacturers will stop offering copay assistance, which, as described above, would severely impact the ability of Americans to afford and access their prescription medications. Additionally, as the Court has clarified, patients will end up paying more for their medications, insurers will be collecting more money, and drug manufacturers will be spending more.
At this time when people are struggling to afford their prescription drugs, among other items to live, nothing must be done to increase their costs.
Covered Drugs Must be Included as Essential Health Benefits
After raising this issue with CMS for several years, we are pleased that steps are finally being taken to clamp down on insurers and employers that are abusing the Affordable Care Act by covering drugs without including them as part of essential health benefits.
CMS is proposing to codify its existing policy, in place since 2016, that plans covering prescription drugs in excess of the state’s benchmark plan are considered essential health benefits (EHB) and therefore are subject to EHB protections, including annual cost-sharing limits. CMS would accomplish this by proposing to amend § 156.122 to add paragraph (f), which would explicitly state that drugs in excess of the benchmark are considered EHB.
In recent years, some insurers and employers have been abusing the system by covering drugs but classifying them as “non-EHB,” and forcing patients to obtain their prescription drugs outside the protections included in the ACA. Payers and vendors who use this scheme also are implementing copay maximizers by exploiting copay assistance from drug manufacturers far in excess of the annual amount payers are entitled to.
Those entities implementing these schemes have not hidden what they are doing.
One of the companies, SaveOnSP, which is working with the PBM, Express Scripts (which is owned by the insurer Cigna), explains on its website in a FAQ titled “How will plan sponsors see savings generated?”
“The plan sponsor will experience savings by leveraging the Affordable Care Act state benchmark requirements to classify certain specialty medications under the category of non-essential health benefits. These medications will not accumulate toward the plan participant’s deductible or out-of-pocket maximums. This allows higher cost-sharing to apply to the medications and a lower overall cost paid by the plan.”
SaveOnSP explains that they help “plan participants save money on their specialty medications by supporting their enrollment in manufacturer copay assistance” and “support[] plan participants on more than 300 medications in approximately 20 therapy classes.”
Express Scripts is very up front on what they are doing and their work with SaveOnSP:
“Patients need their specialty medications, and plans need better affordability. In partnership with SaveOnSP on the first non-essential health benefits copay assistance solution, we’ve driven significant savings by targeting high-cost, high-volume drugs. SaveOnSP utilizes plan-design changes to identify select drugs as non-essential health benefits, enabling maximum savings and reducing plan and member costs.”
The list of drugs that SaveOnSP covers, as stated above, is quite extensive. For 2024 the list can be found here and includes 392 drugs.
That document explains how they operate and that beneficiaries, who already have insurance, must sign up for their program, and then, they will experience reduced costs. If they do not, they will have to pay 30 percent co-insurance. They also state they will seek copay assistance from the drug manufacturer. “By completing the manufacturer copay assistance program’s enrollment process and consenting to SaveOnSP monitoring your pharmacy account, your final cost will be reduced.”
Another company, PrudentRx, which works with the PBM CVS Caremark (which is owned CVS Health, which also owns insurer Aetna), works with self-funded plans and designates as non-essential health benefits over 500 drugs that impact over 50 groups of health conditions. They then force beneficiaries to sign up for their program so that they can extract copay assistance from the drug manufacturers. If they sign up, the cost of the drug would be $0, but if they do not, they would be forced to pay a 30 percent co-insurance.
It is rather ironic that while there are entities (including insurers) that are voicing strong opposition to copay assistance, at the same time they are working with others that are taking advantage of the copay assistance programs and extracting as much as they can for themselves.
In documents sent to beneficiaries enrolled in plans that use PrudentRx, their entire program is fully described: for the Commonwealth of Kentucky, this is what their beneficiaries receive; for the Catholic Diocese of Columbus they receive this explanation; and for the Texas Teachers Retirement System, they receive this explanation.
Insurers also use PrudentRx. PacificSource, which operates in Montana, Oregon, Idaho, and Washington State, uses PrudentRx. In its Health Plans Drug List for Montana, they identify 778 medications as “Prudent” and that “are available on a copay maximizer program, available to select self-insured large groups only.”
Another company called Copay Armor, powered by PillarRx Consulting, according to plan documents in the Highmark Blue Cross Blue Shield system, “helps to leverage manufacturer assistance dollars to lower your prescription out-of-pocket costs. Copay Armor is driven off a unique drug list of high cost mostly specialty medications that utilize manufacturer assistance dollars.” They include a long list of 171 drugs, including numerous HIV and hepatitis drugs.
Variable Copay explains how it works in this manner, “With Variable Copay™, members’ out-of-pocket costs for prescription drugs may be reduced or eliminated by a drug manufacturer’s coupon and the remaining drug coupon dollars are used to offset the costs to the employer.”
We are pleased that the federal government is beginning to crack down on this abuse of the ACA and urge CMS to clarify that the regulation stating that all covered drugs are EHB pertains to all plans that follow EHB, including large group and self-insured plans.
The recent court ruling in the case led by the HIV+Hepatitis Policy Institute striking down the rule that allowed insurers and PBMs not to count copay assistance, which will now prohibit most copay accumulators, in combination with the proposed EHB clarification from CMS, will also eliminate copay maximizers, at least for small group and individual market plans. It is critically important for the federal government to clarify that this also pertains to large group and self-insured plans in order to put an end to copay maximizers.
Alternative Funding Programs
In addition to entities that designate “non-EHB drugs” for the use of extracting manufacturer copay assistance in order to implement copay maximizers, there are other vendors that also use “non-EHB drug” designations to implement alternative funding programs. In these programs, patients who use certain medications are directed to enroll in an alternative program, which is not insurance, in order to bypass ACA laws and regulations relative to patient cost-sharing limits and other patient protections. They then find alternative funding mechanisms to pay for the drugs. If the patient does not comply, they will be left paying the full cost of the drug.
One such company called SHARx used to be more upfront in how it works. While they still state that they are not insurance (thereby bypassing federal and state regulations) and “advocates for the procurement of these medications through many different access points,” they no longer list on their website what those avenues are. However, as we included in our comments on the 2024 NBPP proposed rule, they included, “manufacturer free programs, grants/charities, our International Mail Order Pharmacy partner, domestic wholesale pharmacy and occasionally a copay card.”
Another company, Payer Matrix, works in a similar fashion, and “make[s] 300+ medications available to a wide range of employees and patients” by partnering up with employers and their employees, who are insured, but force them to sign up for Payer Matrix. If they do not, they are forced to pay for the full cost of the drug. The vendor then signs up for drug manufacturers patient assistance programs, which are free drug programs meant for people who are uninsured.
While some of this information is no longer available on their website, it still includes a FAQ titled, “Do I have to provide financial information?” That is answered by the following, “Financial information may be required at times as part of the application process if there is an income threshold requirement for the manufacturer. Not all manufacturers request financials on the application.” This makes it clear what the company is doing.
There are a growing number of other vendors that are working with insurers, employers, and PBMs around the country. In addition to SHARx and Payer Matrix, here is a list that was compiled by Dawn Holcombe of the Connecticut Oncology Association:
https://archimedesrx.com/
https://impaxrx.com
https://www.paydhealth.com/
https://www.rxfree4me.com/#home
https://scriptsourcing.com/
https://www.veracity-benefits.com/veracityrx/ [7]
The federal government must investigate and prohibit these harmful schemes. If legislation is necessary, the administration should work with Congress to pass it.
Prevalence of Plans Using Non-EHB Drug Designations
We are pleased that CMS is finally taking steps to stop these schemes that designate covered drugs as non-EHB. However, we take issue with the statement from CMS that they do not believe this practice is widespread. In response to CMS’ request for input on the extent of these practices occurring, we spent a couple hours conducting searches on the web for plans that use these vendors for their prescription drug coverage. As detailed in Attachment 1, the HIV+Hepatitis Policy Institute compiled a list of over 100 employers across the country including large private companies, universities, state and county governments, unions and non-profits, along with 22 insurers. Not all health plans are made public, particularly those of private employers, so the search results, we assume, are limited.
While the full list is in Attachment 1, employers who are using or have used vendors that are forcing their employees as part of their insurance benefit to enroll with vendors to obtain certain “non-essential health benefit” prescription drugs and then seek copay assistance from drug manufacturers or alternative funding, include the following:
Large Employers: Bank of America, Chevron, Citi, Delta, Hertz, Hilton, Home Depot, NewsCorp, Ruby Tuesday, Target, and United Airlines
States: Connecticut, Delaware, Kansas, Kentucky, and New Mexico
Counties: King County (WA), Mendocino County (CA), Orange County (FL), Sanilac County (MI), San Luis Obispo County (CA)
School Districts: Missouri Educators United Health Plan, Sarasota County Schools, State Teachers Retirement System of Ohio, and Teacher Retirement System of Texas
Universities: Baylor, Carnegie Mellon, Duke, George Washington, Harvard, Kent State, New York University, Ohio State, Purdue, University of California, Yale, and Yeshiva
Unions: New York Teamsters, Screen Actors Guild, Vancouver Firefighters Union, and Writers Guild
Other Non-profits: Catholic Diocese of Columbus, Cleveland Clinic, and Nemours Children’s Health
In addition, we found 22 insurers that include: Blue Cross Blue Shield Massachusetts, Blue Cross Blue Shield of Western New York, Christian Brothers Services, Guidestone Health Insurance, Health Alliance Plan (MI), Johns Hopkins, Medical Mutual of Ohio, Members Health Plan NJ, Premera Blue Cross, Priority Health (MI) and Wellmark BlueCross Blue Shield (Iowa and South Dakota)
While we realize that this quick search does not provide a complete picture, there have been some statistics compiled on the use of maximizers, which utilize the non-EHB scheme.
According to IQVIA, in 2021, 45 percent of covered lives in commercial plans were in plans with maximizers.
Also, according to IQVIA data, presented below, of the $18.7 billion provided annually by copay assistance, $2.6 billion of that was subject to maximizers, which utilize the “non-EHB” scheme (while another $3.5 billion was subject to copay accumulators.)[8]
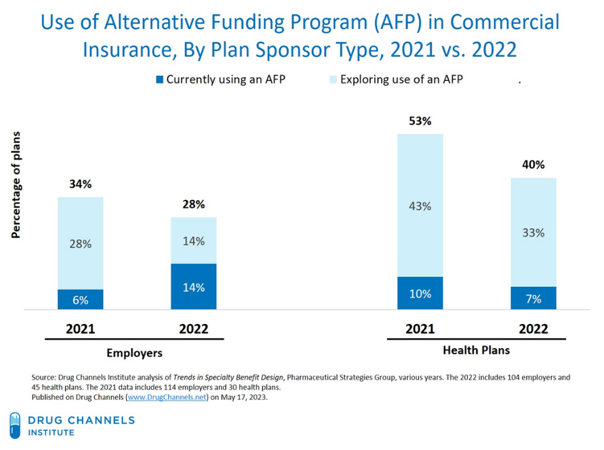
As described in the graph below, according to Adam Fein of Drug Channels, in a survey of employers and plans, in 2022, 14 percent of employers were using Alternative Health Programs, while another 14 percent were exploring their use. Among plans, 7 percent were using them, while another 33 percent were exploring their use.
We believe, based on this data presented, the use of designating covered drugs as non-EHB is extremely widespread and is growing. This practice must be stopped.
Standard Plan Options
We are pleased that CMS is continuing the requirement that issuers offer standardized plan options. These plans utilize copays rather than co-insurance, and for many metal levels costs are pre-deductible. We do, however, believe that these plans fall short in some areas.
First, for some of the metal levels, the maximum allowed copay for specialty-tier drugs is prohibitively high for patients. Co-pays of $250 to $500 or more will lead to beneficiaries abandoning their prescription drugs.
Secondly, for four metal levels, before beneficiaries can take advantage of copayments for drugs on the non-preferred and specialty drug tiers, they still must pay a high deductible.
It is extremely critical that prescription drugs for as many beneficiaries as possible be outside the deductible. Currently, beneficiaries must meet their annual deductible, which is based on the full cost of the list price of the drug and does not consider the substantial amount of rebates insurers and PBMs receive. By including prescription drugs outside the deductible, beneficiaries will be able to better afford and access their medications, particularly at the start of each year, to remain healthy. This would be particularly helpful to beneficiaries with chronic conditions who rely on prescription drugs from one year to the next.
The proposed $7,500 deductible for the Expanded Bronze plans coupled with a $500 copay for Specialty Drugs and a $9,200 annual cost-sharing limit, or a $5,000 deductible in Standard Silver plans coupled with a $350 copay for Specialty Drugs and a $8,000 annual cost-sharing limit, would still make prescription drugs unaffordable for most patients, particularly those with chronic conditions. We strongly urge you to remove all drugs from the deductible and lower the copays for Specialty Drugs from a high of $500 to no more than $100.
We also support the proposal to reduce the number of non-standard plans an issuer can offer, from 4 to 2 for every standard plan offered in that same metal level. We also are supportive of the proposed exceptions process to that limitation if the issuers offers non-standard plans that would benefit consumers with chronic and high-cost conditions, such as HIV and hepatitis C, if patient cost-sharing is 25 percent lower than their corresponding non-standard plan. We applaud CMS for seeking ways to reduce patient cost-sharing, particularly for beneficiaries with chronic and high-cost conditions.
Pharmacy & Therapeutic Committee Standards
The HIV+Hepatitis Policy Institute is highly supportive of the proposed requirement of adding consumer representatives to Pharmacy and Therapeutic Committees beginning in 2026. Adding consumer representatives who have direct experience with the conditions that the drugs are used for in selecting plan formularies will add an important viewpoint and CMS is lauded for this step. HIV+Hep has recently identified a plan in Texas that is blatantly not following HIV treatment guidelines. While P&T committees are already supposed to have representatives with clinical experience, adding a patient perspective should improve compliance and add an important perspective.
We would be interested in learning more about how consumers will be selected, vetted, and compensated. CMS should assemble a task force of internal and external stakeholders to look at best practices and develop these procedures and parameters.
Drug Classification System
We support CMS’ proposal to change the drug classification used to assist in developing EHB from the current United States Pharmacopeia (USP) Medicare Model Guidelines (USP Guidelines) to the USP Drug Classification (USP DC).
As we commented in response to the EHB RFI, the Medicare Model Guidelines were developed for Medicare Part D. Since it was designed to implement Medicare Part D it does not include all classes of drugs, such as those used for weight loss, reproduction, and sexual disorders. It is updated every three years.
On the other hand, USP DC includes more drug classes and is updated annually. Therefore, it incorporates new medical advancements faster for the benefit of patients. USP guidelines dated September 2023 included 46 drug categories, 160 pharmacotherapeutic classes, and listed 2,090 examples of drugs; current USP DC guidelines, dated December 2023, includes 50 categories, 174 classes, and lists 1,991 examples of drugs. The USP DC is developed through USP’s independent, science-based, expert-led process that relies on stakeholder input, including formal public comment periods. The USP Drug Classification Subcommittee comprises academicians, practitioners, formulary experts, patient advocates, and clinicians.
Since it is more inclusive of drug classes relevant to the private insurance patient base and updated more frequently, we are in full support of CMS using the USP DC. We urge CMS to move as quickly as possible to switch to the new system, which we understand will take some time.
We thank you for the opportunity to share these comments and look forward to working with you as you seek to make healthcare more affordable and accessible for more Americans. Should you have any questions or comments, please feel free to contact me at cschmid@hivhep.org. Thank you very much.
Sincerely,

Carl E. Schmid II
Executive Director
Attachment
[1] “The Use of Medicines in the U.S. 2022: Usage and Spending Trends and Outlook to 2026,” IQVIA Institute, April 2022, https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/the-use-of-medicines-in-the-us-2022/iqvia-institute-the-use-of-medicines-in-the-us-2022.pdf, page 40.
[2] “The Use of Medicines in the U.S. 2022,” page 41.
[3] Sara R. Collins, Shreya Roy, Relebohile Masitha. “Paying for It: How Health Care Costs and Medical Debt Are Making Americans Sicker and Poorer,” Findings from the Commonwealth Fund 2023 Health Care Affordability Survey, October 26, 2023, https://www.commonwealthfund.org/publications/surveys/2023/oct/paying-for-it-costs-debt-americans-sicker-poorer-2023-affordability-survey?check_logged_in=1&utm_source=newsletter&utm_medium=email&utm_campaign=newsletter_axiosvitals&stream=top.
[4] “Plan Year 2024 Qualified Health Plan Choice and Premiums in HealthCare.gov Marketplaces,” CMS, last modified 10/25/23, https://www.cms.gov/files/document/2024-qhp-premiums-choice-report.pdf.
[5] “National Health Expenditure Data,” CMS, last modified 12/13/23, https://www.cms.gov/data-research/statistics-trends-and-reports/national-health-expenditure-data/nhe-fact-sheet#:~:text=NHE%20grew%204.1%25%20to%20%244.5,18%20percent%20of%20total%20NHE.
[6] “The Use of Medicines in the U.S. 2022,” IQVIA Institute, April 2022, https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/the-use-of-medicines-in-the-us-2022/iqvia-institute-the-use-of-medicines-in-the-us-2022.pdf, page 47.
[7] Dawn Holcombe, “Understand the Nuances and Impact of Alternate Funding Programs,” National Oncology State Network (PowerPoint Presentation, Copay, Reimbursement and Access Congress, 10/2/23).
[8] Luke Greenwalt, “Summiting the Next Decade—Finding Success on a Difficult Climb,” IQVIA Market Access Center of Excellence (PowerPoint presentation, Payers, Patients, Pharmacies, & Pressure: State of the Payer 2023, 5/1/23).
Attachment 1
Plans & Issuers Using “Non-EHB” Prescription Drug Vendors
Compiled by HIV+Hepatitis Policy Institute
January 8, 2024
- Private sector employers
- BAE Systems https://info.caremark.com/oe/baesystems (PrudentRx)
- Bank of America https://www.bankofamerica.com/content/documents/employees/abe_aug_2024_announcement_article.pdf (Prudent Rx)
- Chevron https://hr2.chevron.com/-/media/hr2/document-library/smm/smm_2023_rxexpressscripts_saveonsp_medppo_final.pdf (SaveOnSP)
- Citibank https://www.citibenefits.com/Health/Prescription-Drugs (PrudentRx)
- Delta Airlines https://deltabenefits.com/# (Prudent Rx)
- Hertz https://hertzbenefits.com/wp-content/uploads/UMR-SPD-2020-2021.pdf (Archimedes)
- Hilton https://cache.hacontent.com/ybr/R516/01250_ybr_ybrfndt/downloads/HiltonUSSPDEnglish.pdf (Prudent Rx)
- Home Depot https://www.caremark.com/portal/asset/HomeDepot_Base_BAAG.pdf (Prudent Rx)
- JCrew https://mybenefits.aon.com/getmedia/2904e963-11e4-461b-aa40-e583cf498074/2023_Benefits_Guide.pdf (ImpaxRx)
- Marathon Petroleum Company https://www.mympcbenefits.com/Documents/MPC-2023-Saveon-Program-Medication-List-Classic-Plan.pdf (SaveOnSP)
- Montana’s Credit Unions https://www.mcun.coop/wp-content/uploads/2023/04/MCULSmithRx_Connect-Patient_Assistance_Program.pdf (Smith Rx)
- NewsCorp https://mynewscorpbenefits.com/news-corp/prudentrx-opportunities-to-save-on-specialty-rx/ (Prudent Rx)
- Potlatch #1 Financial Credit Union (Idaho) https://fliphtml5.com/nwekg/uawj/basic (Payer Matrix)
- Publicis Group https://www.publicisconnections.com/Health-Benefits/-/media/Mercer/Publicis/Documents/PrudentRx_Specialty_Medication_Copay_Program_FAQs.pdf (Prudent Rx)
- Ruby Tuesday https://benefits.rubytuesday.com/pdf/PayerMatrixMemberLeaveBehind_100122_vF.pdf (Payer Matrix)
- Target https://www.express-scripts.com/files/hub/art/open_enrollment/TargetBenOverview.pdf (SaveOnSP)
- Truist Financial https://benefits.truist.com/benefits/pharmacy (Prudent Rx)
- United Airlines https://cache.hacontent.com/ybr/R516/00245_ybr_ybrfndt/downloads/254455.pdf (Prudent Rx)
- States
- Connecticut https://carecompass.ct.gov/wp-content/uploads/2022/04/PrudentRx_OptOut.pdf (Prudent Rx)
- Delaware https://dhr.delaware.gov/benefits/cvs/documents/prudentrx/faq.pdf (Prudent Rx)
- Iowa https://das.iowa.gov/media/3883/download?inline= (Prudent Rx)
- Kansas https://sehp.healthbenefitsprogram.ks.gov/media/cms/2024_Enrollment__Presentation__09_d17de44190c92.pptx (Prudent Rx)
- Kentucky https://extranet.personnel.ky.gov/KEHP/PrudentRx%20Overview.pdf (Prudent Rx)
- New Mexico https://www.mybenefitsnm.com/documents/CVS_and_HR_Reminders.pdf (Prudent Rx replacing SaveOnSP)
- West Virginia Public Employees Insurance Agency https://peia.wv.gov/forms-downloads/prescription-drug-benefits/Pages/default.aspx (SaveOnSP)
- Counties
- Cherokee County GA https://cherokeecountyga.gov/Human-Resources/_resources/documents/US-Rx%20Care_Prescription%20Drug%20Navigation%20Guide%20-%20SS%20w%20International%202023_v.1.pdf (Script Sourcing, importation)
- Clermont County OH https://hr.clermontcountyohio.gov/prescription-plan/ (Payd Health)
- Dunn County WI https://vendornet.wi.gov/Download.aspx?type=bid&Id=54a11329-5ad2-ed11-9043-00505684483d&filename=Appendix+B+-+2023+Dunn+County+Benefit+Booklet.pdf (Scout Rx)
- King County WA https://kcemployees.com/2023/12/18/save-100-on-specialty-medications-with-prudentrx/ (Prudent Rx)
- Mendocino County CA https://www.mendocinocounty.org/government/executive-office/health-insurance-plan/prescription-coverage (SaveOnSP)
- Orange County FL https://www.orangecountyfl.net/Portals/0/resource%20library/employment%20-%20volunteerism/2024MedicalBenefits/SaveOnSP.pdf (SaveOnSP)
- Sanilac County MI https://www.sanilaccounty.net/Handlers/File.ashx?ID=204727 (SHARx)
- San Luis Obispo County CA https://www.slocounty.ca.gov/Departments/Human-Resources/Employee-Benefits/Pharmacy/Pharmacy-Benefits/SaveOnSP.aspx (SaveOnSP)
- Summit County OH https://hreb.summitoh.net/files/6519/meeting_file/openenrollmentguide.pdf (SaveOnSP, ImpaxRx)
- Tehama County CA https://www.co.tehama.ca.us/wp-content/uploads/2022/04/Save-on-SP.pdf (SaveOnSP)
- Tulare County CA https://tularecounty.ca.gov/hrd/benefits-wellness/pharmacy/variable-co-pay-assistance-program/ (EmpiRx Variable Copay Assistance Program)
- Waukesha County WI https://www.waukeshacounty.gov/globalassets/administration/human-resources/benefits/true-rx-spd-waukesha-county-002.pdf (ShaRx)
- Cities and other local jurisdictions
- Cheyenne WI https://cheyenne.granicus.com/MetaViewer.php?view_id=5&event_id=1142&meta_id=123138 (Payd Health)
- Columbus GA https://www.columbusga.gov/Portals/HR/pdfs/DPS%20Flyer.pdf (ImpaxRx)
- New Jersey State League of Municipalities https://www.njlm.org/DocumentCenter/View/8097/111919-0345-rxcostdriverspart2 (SaveOnSP)
- Village of Lake Zurich IL https://lakezurich.org/DocumentCenter/View/11785/SaveonSP-Overview-and-FAQs?bidId= (SaveOnSP)
- School Districts and Teacher Retirement Plans
- Arizona School Boards Association Insurance Trust https://content.myconnectsuite.com/api/documents/72c88ea8876746b7b21a1b51edeb43a1.pdf (PrudentRx)
- Clovis Unified School District https://www.cusd.com/Downloads/PrudentRx%20Program%20Specialty%20Drug%20Complete%20List%20(1).pdf (Prudent Rx)
- Menasha Joint School District WI https://doa.wi.gov/School%20District%20Health%20Ins%20Attachments/2021-22%20Menasha%20Joint%20Benefits%20Summary.pdf (ScoutRx)
- Missouri Educators Unified Health Plan (MEUHP) http://meuhp.com/media/20100/saveonsp%20broker%20client%20flyer.pdf (SaveOnSP; Cigna Plan)
- Osceola County FL School District https://www.osceolaschools.net/cms/lib/FL50000609/Centricity/Domain/156/ElectRx%20International%20Mail%20Order%20Program.pdf (Elect Rx, importation)
- Pinellas County Schools FL https://www.pcsb.org/Page/37275 (Prudent Rx)
- Ripon Area School District (WI) https://www.ripon.k12.wi.us/cms_files/resources/2023%20Benefit%20Guide%20(5).pdf (Scout Rx)
- Sarasota County Schools https://www.sarasotacountyschools.net/cms/lib/FL50000189/Centricity/Domain/1148/High%20PPO%20Plan%203769%20RX%20SBC%201-1-2024%20Rev.%20FINAL.pdf (SaveOnSP)
- State Teachers Retirement System of Ohio https://www.strsoh.org/_pdfs/health-care/saveonsp.pdf (SaveOnSP)
- Teacher Retirement System of Texas https://www.trs.texas.gov/TRS%20Documents/faq-prudentrx.pdf (Prudent Rx)
- Universities
- Barton Community College https://docs.bartonccc.edu/humres/HRBenefits%20and%20Discounts/Benefits/Health%20Plan%20Open%20Enrollment%20Links/BCCC%20Payer%20Matrix%20Overview%20%20FAQs%20-Combined%20(002).pdf (Payer Matrix)
- Baylor University https://hr.web.baylor.edu/sites/g/files/ecbvkj1046/files/2023-03/prudentrx_copay_optimization.pdf (Prudent Rx)
- Brown University https://www.brown.edu/about/administration/human-resources/benefits/health-and-wellbeing/prescription-drug-coverage (Pillar Rx)
- Butler University https://www.butler.edu/wp-content/uploads/sites/14/2022/01/paydhealth_select_drugs_and_products_program_member_mrx1346_0420-3.pdf (Payd Health)
- Carnegie Mellon University https://www.cmu.edu/hr/benefits/health-welfare/prescription/prudent-rx.html (Prudent Rx)
- Duke University https://hr.duke.edu/benefits/medical/pharmacy/ (SaveOnSP)
- George Washington University https://hr.gwu.edu/prudent (Prudent Rx)
- Harvard Universityhttps://hughp.harvard.edu/prescriptions (Pillar Rx)
- Hendrix College https://www.hendrix.edu/uploadedFiles/Campus_Resources/Human_Resources/Benefits_Info/2024%20Health%20Benefit%20Overview.pdf (Payer Matrix)
- Illinois Institute of Technologyhttps://www.iit.edu/sites/default/files/2021-07/prudent-rx-participant-qa.pdf (Prudent Rx)
- Iona University https://www.iona.edu/offices/human-resources/employee-benefits/health-insurance/saveonsp-variable-copayments-certain (SaveOnSP)
- Ithaca College https://www.ithaca.edu/intercom/2023-11-03-prudent-rx-update-and-open-enrollment-resources (use of Prudent Rx paused)
- Kent State University https://www.kent.edu/hr/benefits/prescription-cvs-health (Prudent Rx)
- Loyola University of New Orleans https://finance.loyno.edu/sites/default/files/2023-10/2024%20Loyola%20Benefits%20Guide.pdf (Payer Matrix)
- Missouri Southern State Universityhttps://www.mssu.edu/business-affairs/human-resources/2023-MSSU_Benefit-Guide_FULL-TIME_20230101.pdf (Payer Matrix)
- Northwestern University https://hr.northwestern.edu/benefits/health-insurance/health-insurance-plans/prescription-drug-benefits/ (SaveOnSP)
- New York University https://www.nyu.edu/employees/benefit/full-time/professional-research-staff/benefits-guide-2023/prescription-drug-plan/prudentrx-specialty-medication-program.html (Prudent Rx)
- Northwestern University https://hr.northwestern.edu/benefits/health-insurance/health-insurance-plans/prescription-drug-benefits/ (SaveOnSP)
- Oakland University https://www.oakland.edu/Assets/Oakland/uhr/files-and-documents/2022-Benefits/2022%20OU%20Guide%20Faculty_final.pdf (Pillar Rx)
- Ohio University https://www.ohio.edu/hr/benefits/prescription-drug-coverage (Prudent Rx)
- Ohio State University https://hr.osu.edu/wp-content/uploads/rx-saveonsp-list.pdf (SaveOnSP)
- Princeton University https://hr.princeton.edu/sites/g/files/toruqf1976/files/documents/2022-SPD-prescription-drug-plan.pdf (OptumRx Variable Copay Solution)
- Purdue University https://www.purdue.edu/hr/Benefits/prescription/ (Archimedes)
- Texas A&M Universityhttps://www.tamus.edu/business/prescription-programs-and-your-am-system-prescription-drug-benefits/ (SaveOnSP)
- University of Alaska https://www.alaska.edu/hr/benefits/documents-and-forms/pharmacy/2021saveonsp-drug-list.pdf (SaveOnSP)
- University of Californiahttps://ucnet.universityofcalifornia.edu/forms/pdf/2023_uchsp-rx-booklet.pdf (Lumicera/Navitus)
- University of Connecticut https://hr.uconn.edu/wp-content/uploads/sites/1421/2022/05/2022-SEBAC-Agreement.pdf (Prudent Rx)
- University of Pittsburgh https://www.hr.pitt.edu/sites/default/files/PrescriptionDrugFAQ.pdf (SaveOnSP)
- University of Richmondhttps://hr.richmond.edu/benefits/insurance/medical-plans/pdf/SaveonSP.pdf (SaveOnSP)
- University of Wisconsin https://www.wisconsin.edu/ohrwd/benefits/health/pharmacy-benefits/ (Navitus/Lumicera)
- Villanova University https://www1.villanova.edu/content/dam/villanova/hr/documents/SBC-23-24%20PPO%20Villanova%20University.docx (SaveOnSP)
- Washington University in St. Louis https://hr.wustl.edu/benefits/medical-dental-life/prescription-drug-benefit/ (SaveOnSP)
- Yale University https://your.yale.edu/work-yale/benefits/benefits-enrollment-2024/managerial-and-professional-benefits-2024 (Prudent Rx)
- Yeshiva https://www.yu.edu/sites/default/files/inline-files/Yeshiva%202021%20OE%20Presentation_Final%20%28003%29.pdf (Prudent Rx)
- Unions
- ATU 1181 (NY) https://atu1181.org/wp-content/uploads/2019/04/Active-SBC-4-15-19-12-31-19.pdf (Payer Matrix)
- Food Employers Labor Relations Association and United Food and Commercial Workers VEBA Fund https://www.associated-admin.com/images/pdf/FELRA/FELRA%20SMM%20re%20COVID-19%20and%20SaveOn%203.23.2020.pdf (SaveOnSP)
- International Association of Machinists and Aerospace Workers https://www.iambtf.org/medical-prescriptions/prudentrx-copay-program (Prudent Rx)
- New York Teamsters https://nytfund.org/media/jdedv5pz/20211122-saveonsp-drug-list-effective-01012022.pdf (SaveOnSP)
- Screen Actors Guild https://www.sagaftraplans.org/health/cvs-specialty (Prudent Rx)
- Sprinkler Fitters of Chicago https://sprinklerfitterchicago.org/ULWSiteResources/ualocal281_v2/Resources/file/health-welfare/documents/smm-21.pdf (Payd Health)
- Tri-County Building Trades Health Fund (MI, OH, WV) https://www.ourbenefitoffice.com/SheetMetalWorkers33/Benefits/Module/Member/MaintFileUploadPopup.aspx?fileUploadID=zLAQ7vZg2Rs%3D (Payd Health)
- Vancouver Firefighters Union (WA) https://www.vanfiretrust.org/payer-matrix.html (Payer Matrix)
- Writers Guild https://www.wgaplans.org/saveonsp/ (SaveOnSP)
- Other non-profit organizations
- Broward Health, FL https://employee.browardhealth.org/-/media/broward-health/employee/benefits/prudentrx-frequently-asked-questions.pdf (Prudent Rx)
- Catholic Diocese of Columbus https://columbuscatholic.org/system/resources/W1siZiIsIjIwMjEvMTAvMTkvMWxrM2diNjFkeV9BRVROQV9QcnVkZW50X1J4X0FtZW5kbWVudF85LjEuMjEucGRmIl1d/AETNA%20Prudent%20Rx%20Amendment%209.1.21.pdf (Prudent Rx)
- Cleveland Clinic https://employeehealthplan.clevelandclinic.org/Home/Resources/Specialty-Drug-Copay-Card-Assistance-Programs (Prudent Rx)
- Nemours Children’s Health https://nemoursbenefitsguide.com/wp-content/uploads/2022/12/2023-Rx-Plan-overview-Nemours.pdf (SaveOnSP)
- Lake Metropolitan Housing Authority https://digital.nfp.com/vlp/Lake%20Metropolitan%20Housing%20Authority%20Landing%20Page (ImpaxRx)
- Issuers
- Advantage Health Plans (TX, OK) https://www.advantagehealthplans.com/pdf/AHP%20Southern%20Scripts%20Variable%20Copay.pdf (Southern Scripts Variable Copay Program)
- Blue Cross Blue Shield of Kansas https://benefits-direct.com/ottawa290/wp-content/uploads/sites/81/2023/09/FlexAccess-member-flyer-2023.pdf (FlexAccess)
- Blue Cross Blue Shield of Massachusetts https://home.bluecrossma.com/collateral/sites/g/files/csphws1571/files/acquiadam-assets/Cost%20Share%20Assistance%20Medication%20List.pdf (Pillar Rx)
- Blue Cross Blue Shield of Michigan https://www.bcbsm.com/amslibs/content/dam/public/employers/documents/share-resources-employees/individual-files/high-cost-drug-discount-program.pdf (Pillar Rx)
- Blue Cross Blue Shield of Minnesota https://www.bluecrossmn.com/sites/default/files/DAM/2022-09/2023_RX-Fact-Sheet_AGCS%2BMedsYourWay_91922.pdf (FlexAccess)
- Blue Cross Blue Shield of Nebraska https://www.nebraskablue.com/-/media/Files/NebraskaBlueDotCom/Shop-Plans/Group-Health-Plans/Large-Group-Plans/PremierBlue_Plan_Options_92106.pdf (FlexAccess)
- Blue Cross Blue Shield of Western New York https://www.bcbswny.com/content/dam/BCBSWNY/broker-group/public/pdf/group/computer-task-group/Saveon-Member-Flyer.pdf (SaveOnSP)
- Chorus Community Health Plans (Wisconsin) https://chorushealthplans.org/getmedia/87931284-9823-4cef-b6b0-75fba6b587ec/Chorus-Gold-SOB-2024-(Rev-2023-06-12).pdf (SaveOnSP)
- Christian Brother Services https://www.cbservices.org/assets/images/health/health_benefit_flyers/H&B_SaveonSP%20Program.pdf (SaveOnSP)
- EMI Health (offers medical insurance to corporate, government, public education, and higher education groups in Arizona, Georgia, Texas, and Utah) https://emihealth.com/pdf/saveon.pdf (SaveOnSP)
- Guidestone Health Insurance https://www.guidestone.org/-/media/Insurance/LifeConversionForms/Express-Scripts-SaveonSP-Medication-List (SaveOnSP)
- Health Alliance Plan (MI) https://www.hap.org/-/media/project/hap/hap/files/hap/prescription/2024/2024-copay-assistance-program-for-hap-members-flyer.pdf (SaveOnSP)
- Johns Hopkins https://www.hopkinsmedicine.org/johns-hopkins-health-plans/providers-physicians/our-plans/ehp/pharmacy-formulary (Prudent Rx)
- Medical Mutual of Ohio https://www.medmutual.com/-/media/88221371697746DA9E847850C2B8754A.ashx?h=16&thn=1&w=16 and https://www.buaweb.com/files/63123/the_file/saveonsp_flyer_c3116rxx_422.pdf (SaveOnSP)
- Members Health Plan NJ https://membershealthplannj.com/wp-content/uploads/2019/12/2019-SaveOn-list.pdf (SaveOnSP)
- Network Health Insurance Plans (Wisconsin) https://networkhealth.com/__assets/pdf/pharmacy/saveon-drug-list.pdf (SaveOnSP)
- Pacific Source Health Plans (MT, OR, ID, WA) https://pacificsource.com/ps_find_drug/pdf/MT/2023 (Prudent Rx copay maximizer, see p2)
- Premera Blue Cross https://www.premera.com/documents/052560.pdf (SaveOnSP)
- Priority Health (MI) https://www.priorityhealth.com/individual-family-health-insurance/learning-center/mypriority-plan-benefits (SaveOnSP)
- University of Pittsburgh Medical Center Plans https://www.upmchealthplan.com/aon/pharmacy.aspx (SaveOnSP)
- Wellmark BlueCross Blue Shield (Iowa and South Dakota) https://www.wellmark.com/-/media/sites/public/files/member/prudentrx-drug-list.pdf?sc_lang=en&hash=D71214E333698A85351498D5E6CB4D57 (Prudent Rx)
- Wisconsin Physicians Service Insurance Corporation https://secure.wpsic.com/sales-materials/files/35618-wps-aso-esi-specialty-drug-program.pdf (SaveOnSP)