Letter to Tamara Jensen on Medicare NCD Update for Hepatitis C Screening

![]()

Tamara Syrek Jensen, JD
Director, Coverage and Analysis Group (CAG)
Center for Clinical Standards and Quality (CCSQ)
Centers for Medicare and Medicaid Services (CMS)
7500 Security Blvd.
Baltimore, MD 21244
Re: Medicare NCD Update for Hepatitis C Screening
Dear Ms. Jensen:
We are writing on behalf of providers, community-based organizations, advocates, and consumers who work on or are affected by the hepatitis C virus (HCV). In order to ensure that Medicare coverage requirements align with clinical and public health best practices, we urge CMS to update the HCV screening National Coverage Determination (NCD) to bring it in line with the 2020 U.S. Preventive Services Task Force (USPSTF) recommendation and the 2020 Centers for Disease Control and Prevention (CDC) screening recommendations.
In 2016, CDC estimates that 2.4 million people were living with HCV in the United States.[1] And yet between 2013 and 2016, only 60 percent of individuals living with HCV were even aware of their status.[2] Despite the increasing incidence of HCV, particularly among people who inject drugs, ensuring access to and reimbursement by Medicare for HCV screening remains a challenge. Increasing the number of people who know their HCV status is also critical to ending HCV in this country.
The Viral Hepatitis National Strategic Plan–which was released in January 2021–calls for the elimination of viral hepatitis as a public health threat by 2030. The plan includes a focus on hepatitis prevention and treatment activities, including a charge to scale up HCV testing, particularly in community-based settings. Objective 2.1 of the plan calls for an increase of “the proportion of people who are tested and aware of their viral hepatitis status.” This includes scaling up implementation of universal HCV screening guidelines among all adults.
HCV testing is also essential to linking people to curative therapy, another critical goal of the plan (see Objective 2.3.2 calling to ‘[e]xpand hepatitis C screening and treatment capacity among public health, primary care and other health care providers, including pharmacists, to support the implementation of viral hepatitis testing, counseling, and treatment recommendations”). Ultimately, the goal is to increase cleared HCV infection to 58% by 2025 and 80% by 2030. It is critical that Medicare coverage requirements align with the universal testing and linkage to treatment goals of the Viral Hepatitis National Strategic Plan.
Recognizing the benefits of increasing access to HCV screening, the USPSTF updated its recommendation for HCV screening in 2020.[3] In its updated recommendation, USPSTF gave HCV screening a “B” grade for all adults aged 18-79, regardless of symptoms or risk group. The USPSTF clarified in its practice considerations that all adults should be offered a one-time screening, and those at increased risk (e.g., people who inject drugs) should be screened more regularly. This 2020 recommendation replaced the previous 2013 recommendation, which had only recommended screening for those at increased risk of HCV and one-time screening for those in the “baby boomer” cohort (people born between 1945 and 1965). Similarly, the CDC also updated its recommendations in 2020 to include universal screening for adults and periodic screening for those at high risk of HCV.[4] However, the Medicare NCD issued by CMS in 2014 remains outdated and does not reflect the latest evidence and clinical considerations.[5]
We urge Medicare to update the current NCD, and as part that review to consider the following:
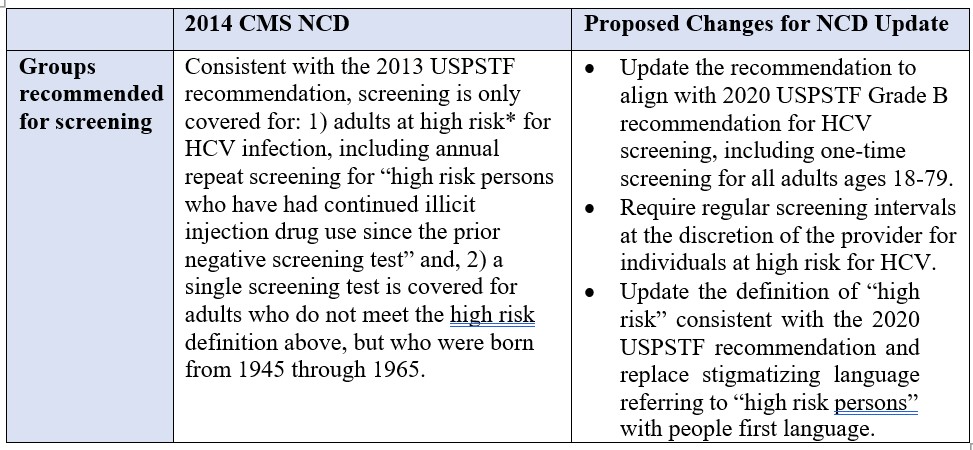
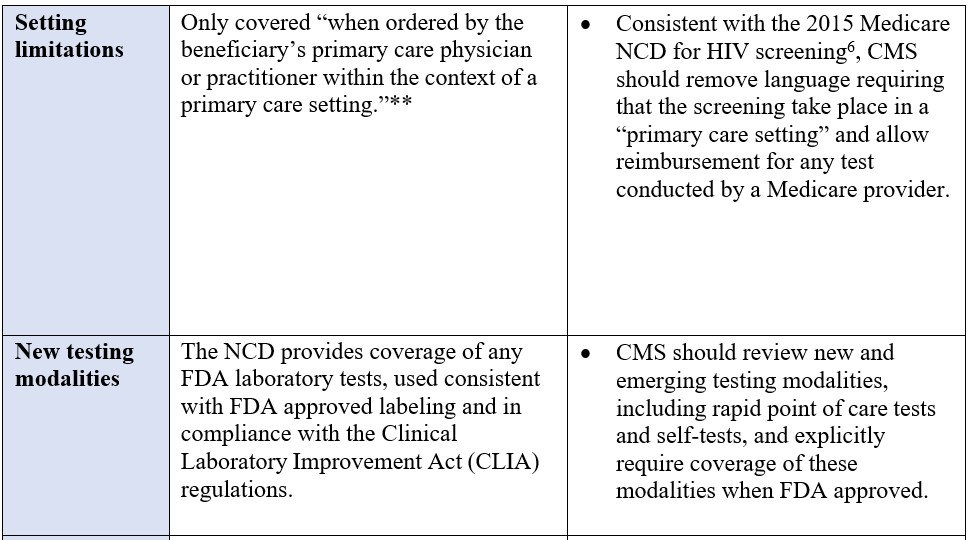
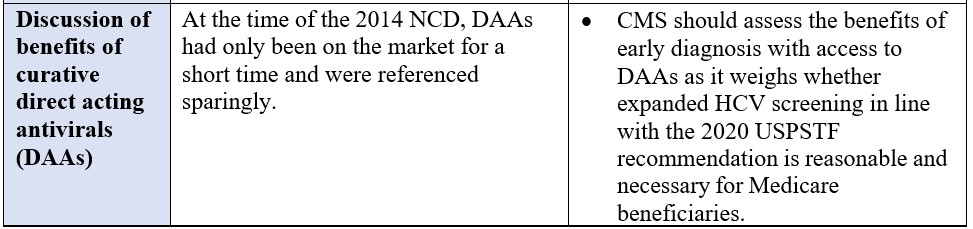
*“High risk” is defined as persons with a current or past history of illicit injection drug use; and persons who have a history of receiving a blood transfusion prior to 1992.
**The definition of primary care setting explicitly excludes: emergency departments, inpatient hospital settings, ambulatory surgical centers, independent diagnostic testing facilities, skilled nursing facilities, inpatient rehabilitation facilities, clinics providing a limited focus of health care services, and hospice.
In addition to considering the changes discussed above, we urge CMS to review the evidence base that has emerged since the 2014 NCD. The bulk of this evidence has already been compiled as part of the 2020 USPSTF process and the 2020 CDC HCV screening recommendation update, both discussed and cited above. The key areas emerging from the data and studies compiled by USPSTF and CDC include the following:
- Benefits of early detection and treatment
The USPSTF reviewed clinical studies assessing the impact of DAA access on sustained virologic suppression (SVR) and found that modeling studies that compared screening in all persons 18 years and older with screening just for the baby boomer birth cohort suggested that “expanded screening strategies would be beneficial, despite different assumptions regarding chronic HCV infection progression and rates of linkage to care.” - Harms of screening or treatment
The USPSTF reviewed newer studies finding that DAA regimens were associated with fewer harms than older interferon-containing therapies and had a shorter treatment duration length. - Defining those at “high risk” for HCV
Public comments urged USPSTF to provide more specificity around communities and individuals at “high risk” for HCV and what interval of screening is clinically optimal for each population. This is an area that USPSTF addressed in its Practice Considerations section, but warrants additional attentions and review of the evidence. The 2020 CDC HCV screening recommendations go into additional detail with regard to those at high risk and appropriate screening intervals.
Medicare is a critical program, not only for individuals over the age 65 (many of whom are baby boomers and at an increased risk for having HCV), but also for younger adults with Medicare coverage based on a disability determination or who are dually eligible for Medicaid and Medicare. We appreciate your time and attention to this important issue and look forward to learning more about the process CMS is undertaking to ensure that its HCV NCD reflects the most up-to-date clinical considerations and public health guidelines.
We look forward to meeting with you to further discuss this request. If you have any questions or comments, please contact Carl Schmid, HIV+Hepatitis Policy Institute at cschmid@hivhep.org or (202) 462-3042.
Sincerely,
HIV+Hepatitis Policy Institute
National Viral Hepatitis Roundtable
Caring Ambassadors
NASTAD
The AIDS Institute
1 CDC, Hepatitis C Information, available at https://www.cdc.gov/hepatitis/hcv/cfaq.htm#A7.
2 Department of Health and Human Services (HHS), Viral Hepatitis National Strategic Plan (January 2021), available at https://www.hhs.gov/sites/default/files/Viral-Hepatitis-National-Strategic-Plan-2021-2025.pdf.
3 USPSTF, Hepatitis C Virus Infection in Adolescents and Adults: Screening (March 2020), available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening.
4 CDC, CDC Recommendations for Hepatitis C Screening Among Adults — United States, 2020 (April 2020) available at https://www.cdc.gov/mmwr/volumes/69/rr/rr6902a1.htm.
5 CMS, National Coverage Determination for Screening for Hepatitis C Virus (HCV) in Adults (2014), available at https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=361. .
6 CMS National Coverage Determination for Screening for Human Immunodeficiency Virus (HIV) in Adults (2015), available at https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=335&ncdver=2&bc=AAAAEAAAAAAA&