Letter to USPSTF requesting an update of PrEP recommendations to include long-acting agents

5600 Fishers Lane
Mail Stop 06E53A
Rockville, MD 20857
Dear USPSTF Members:
We are writing on behalf of HIV providers, community-based organizations, public health practitioners, and people living with and at increased risk for HIV. We urge the U.S. Preventive Services Task Force (USPSTF) to quickly and efficiently evaluate long-acting cabotegravir, a long-acting injectable form of pre-exposure prophylaxis (PrEP), as part of your recommendation for the prevention of HIV.
PrEP is a unique preventive intervention both because of its safety and efficacy as well as the rapidity at which innovative PrEP products are coming to market. The current USPSTF recommendation for PrEP was released in 2019 (providing a Grade A recommendation for PrEP “with effective antiretroviral therapy to persons who are at high risk of HIV acquisition”).[1] At this time, only one administration route for PrEP had been approved by the Food and Drug Administration (FDA), an oral once daily pill-based antiretroviral (ARV) regimen. In the next six months, however, a long-acting injectable form of PrEP (long-acting cabotegravir) will likely be approved by the FDA. At the core of the federal Ending the HIV Epidemic initiative is an ambitious goal to drastically reduce new HIV transmission by 90 percent by 2030. Increasing the uptake of PrEP is a key component to ending HIV in the United States.
It is imperative that USPSTF recommendations reflect the current science and clinical practice guidelines so that providers are aware of these important innovations in the PrEP landscape and able to advise patients on the PrEP regimen that will be best for each patient. Ensuring providers are up-to-date on the latest clinical recommendations for PrEP is particularly important given the persistent gaps in providers aware of and willing to prescribe PrEP in the United States.[2]
PrEP is both an incredibly effective and woefully underutilized prevention intervention in the U.S. The two oral anti-retroviral (ARV) therapies approved for PrEP in the U.S.–emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) and emtricitabine/tenofovir alafenamide (FTC/TAF)–are over 90 percent effective at preventing sexual transmission of HIV and over 70 percent effective at preventing HIV transmission among people who inject drugs.[3] At the same time only ~20 percent of people indicated for PrEP have actually been prescribed PrEP.[4]
There are also alarming demographic disparities in who is aware of and prescribed PrEP. Despite HIV incidence being significantly higher among Black and Hispanic gay and other men who have sex with men (MSM), white MSM are significantly more likely than Black and Hispanic MSM to report PrEP awareness, discussion with a health care provider, and use.[5] The most recent Centers for Disease Control and Prevention (CDC) data on PrEP utilization reflects these widening disparities. In 2019, 63% of White Americans recommended for PrEP received a prescription for the medications, compared to 14% of Latinx/Hispanic Americans and just 8% of Black Americans.[6]
New modalities of PrEP–including long-acting injectable formulations–have the potential to increase access to PrEP, particularly for individuals for whom adherence to a daily oral pill regimen is difficult.
In November 2020, ViiV Healthcare was granted breakthrough designation by the FDA for long-acting cabotegravir based on early clinical trial success.[7] In May 2021, ViiV Healthcare initiated a rolling new drug application to the FDA for long-acting cabotegravir for the prevention of HIV, with anticipated approval as early as the end of 2021.[8] And on September 28, 2021, ViiV announced the FDA had accepted and granted Priority Review for its NDA, with a target approval date of January 24, 2022.[9] The FDA application is based on the overwhelmingly successful results of two clinical trials. HPTN 083[10] evaluated the safety and efficacy of long-acting cabotegravir for HIV prevention in MSM and transgender women, and HPTN 084[11] evaluated long-acting cabotegravir for HIV prevention in women who are at increased risk of HIV acquisition. The results of HPTN 083 have been published in a peer reviewed journal and the publication of the HPTN 084 is imminent.[12]
Below are summaries of the two trials:
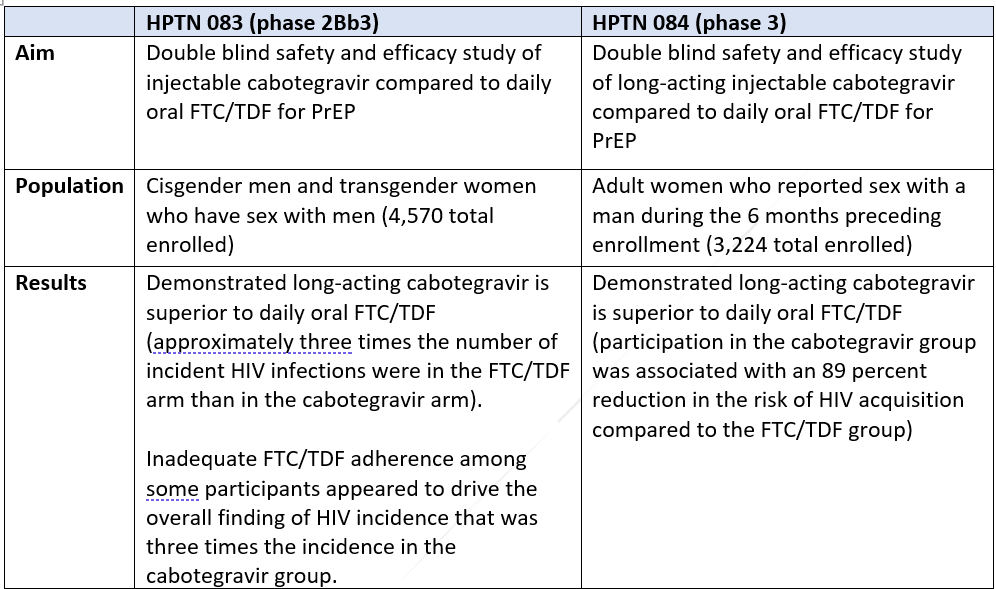
Results of both studies conclude that long-acting cabotegravir is superior to daily oral FTC/TDF. Researchers concluded that better adherence among the trial participants given long-acting cabotegravir compared to those given oral FTC/TFD was the chief driver of the overall finding that long-acting cabotegravir reduced HIV incidence. Given these extremely positive conclusions, it is imperative to populations at increased risk for HIV in this country for USPSTF to update its current recommendation since the clinical evidence available now supports expanded access to new PrEP modalities.
The CDC released updated draft PrEP guidelines in May 2021.[13] In that guidance the CDC included long-acting cabotegravir in light of the overwhelming evidence base for this modality and likely rapid FDA approval. The CDC updated the PrEP guidelines to include specific recommendations and clinical considerations for initiation of long-acting cabotegravir for PrEP, including recommended intervals for regular HIV testing, hepatitis testing, STD testing, and metabolic screening to ensure safe and effective use of the product.
USPSTF should similarly initiate an expedited review of this new PrEP formulation and update the current USPSTF recommendation for PrEP to include long-acting injectable products.
If you have any questions or comments, please contact Carl Schmid, HIV+Hepatitis Policy Institute, at cschmid@hivhep.org or (202) 462-3042.
Sincerely,
ADAP Advocacy Association
African American Health Alliance
AIDS Action Baltimore
AIDS Alabama
AIDS Alliance for Women, Infants, Children, Youth & Families
AIDS Foundation Chicago
AIDS United
American Academy of HIV Medicine
American Sexual Health Association
APLA Health
AVAC
Avita Pharmacy
Black AIDS Institute
CAEAR Coalition
Cascade AIDS Project
Center for Health Law and Policy Innovation
Center on Halsted
Chicago Department of Public Health
Community Access National Network (CANN)
Covid Clinic
Equality California
Equality Florida
Fenway Health
Florida HIV AIDS Advocacy Network
Friends for Life
Georgia AIDS Coalition
GLMA: Health Professionals Advancing LGBTQ Equality
Greater Community AIDS Project of East Central Illinois
HealthHIV
HIV+Hepatitis Policy Institute
HIV Dental Alliance
HIV Medicine Association
Howard Brown Health
Human Rights Campaign
Illinois Public Health Association
International Association of Providers of AIDS Care
John Snow, Inc. (JSI)
Latino Commission on AIDS
Latinos Salud
Los Angeles LGBT Center
Lurie Children’s Hospital
Mother and Child Alliance
NASTAD
National Black Gay Men’s Advocacy Coalition
National Coalition for LGBT Health
National Family Planning & Reproductive Health Association
NMAC
North Carolina AIDS Action Network
PlusInc
Positive Impact Health Centers
PrEP4All
Prevention Access Campaign
San Francisco AIDS Foundation
SisterLove, Inc.
Stroger Hospital of Cook County
The AIDS Institute
The Aliveness Project, Inc.
The FL HIV Justice Coalition
The Well Project
TPAN
Treatment Action Group
University of California, San Francisco
Whitman-Walker Institute
[1] USPSTF, Final Recommendation Statement, Prevention of Human Immunodeficiency Virus (HIV) Infection: Preexposure Prophylaxis (June 2019), available at https://www.uspreventiveservicestaskforce.org/home/getfilebytoken/poDYcagnw7SqKNNbrFt_CV.
[2] Kenneth H. Mayer, Allison Agwu, and David Malebranche, Barriers to the Wider Use of Pre-exposure Prophylaxis in the United States: A Narrative Review, Advances in Therapy, 37, pages 1778–1811 (2020),
available at https://link.springer.com/article/10.1007/s12325-020-01295-0.
[3] CDC, PrEP Effectiveness, available at https://www.cdc.gov/hiv/basics/prep/prep-effectiveness.html.
[4] Harris NS, Satcher Johnson A, Huang YA, et al. Vital Signs: Status of Human Immunodeficiency Virus Testing, Viral Suppression, and HIV Preexposure Prophylaxis — United States, 2013–2018. Morbidity and Mortality Weekly Report: Recommendations and Reports. 2019;68(48):1117- 1123, available at https://www.cdc.gov/mmwr/volumes/68/wr/mm6848e1.htm?s_cid=mm6848e1_w.
[5] Kanny D, Jeffries WL IV, Chapin-Bardales J, et al., Racial/Ethnic Disparities in HIV Preexposure Prophylaxis Among Men Who Have Sex with Men — 23 Urban Areas, 2017, Morbidity and Mortality Weekly Report 2019;68:801–806, available at DOI: http://dx.doi.org/10.15585/mmwr.mm6837a2.
[6] CDC, 2019 National HIV Surveillance System Reports (May 2021), available at https://www.cdc.gov/nchhstp/newsroom/2021/2019-national-hiv-surveillance-system-reports.html.
[7] ViiV Healthcare, ViiV Healthcare receives FDA Breakthrough Therapy Designation for investigational, long-acting cabotegravir for HIV prevention, November 17, 2020, available at https://viivhealthcare.com/en-us/us-news/us-articles/2020/viiv-healthcare-receives-fda-breakthrough-therapy-designation-for-investigational-long-acting-cabotegravir/.
[8] ViiV Healthcare, ViiV Healthcare initiates rolling submission of new drug application with US FDA for long-acting cabotegravir for prevention of HIV, May 4, 2021, available at https://viivhealthcare.com/en-gb/media/press-releases/2021/may/viiv-healthcare-initiates-rolling/.
[9] ViiV Healthcare, FDA grants Priority Review to ViiV Healthcare’s New Drug Application for cabotegravir long-acting for prevention of HIV, September 28, 2021, available at https://viivhealthcare.com/en-gb/media/press-releases/2021/september/viiv-healthcare-announces-fda-priority-review/.
[10] HIV Prevention Trials Network, HPTN 083 Study Summary, available at https://www.hptn.org/research/studies/hptn083.
[11] HIV Prevention Trials Network, HPTN 084 Study Summary, available at https://www.hptn.org/research/studies/hptn084.
[12] R.J. Landovitz et al., Cabotegravir for HIV Prevention in Cisgender Men and Transgender Women, NEJM, Vo. 385;7 August 12, 2021.
[13] U.S. Public Health Service, Pre-exposure prophylaxis for the Prevention of HIV Infection in the United States 2021 Update (DRAFT), available at https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf.