Substandard and discriminatory HIV medication coverage and plan design by Community Health Choice Texas

Deputy Administrator and Director
Center for Consumer Information and Insurance Oversight (CCIIO)
Centers for Medicare and Medicaid Services
200 Independence Avenue SW
Washington DC 20201
Dear Dr. Montz:
We are writing to express our continuing concern about substandard, discriminatory coverage of HIV treatment medications on Community Health Choice Texas “Premier” and “Select” health insurance plans. As we have previously stated, the formularies for these plans, among other things, do not meet the regulatory standards for formulary adequacy in CFR 156.122 (a)(3)(iii)(H) by failing to cover treatment regimens specified in broadly accepted treatment guidelines and that are indicative of clinical best practice, and by discouraging enrollment by people living with HIV.[1]
We continue to urge CCIIO, as the entity that reviews, approves, and regulates Marketplace plans in Texas, to take immediate action against Community Health Choice for offering these substandard and discriminatory plans that violate the ACA and its implementing regulations, and to ensure that these violations do not continue in the 2025 plan year in these and other plans throughout the country.
We first wrote to CCIIO in September 2023 with a formal complaint concerning inadequate coverage and adverse tiering of HIV treatment medications by Community Health Choice Texas. After we received a response from CCIIO in December 2023 that did not address our concerns, we described them further in comments on the 2025 draft Letter to Issuers.
After our complaints and media attention, in March 2024, Community Health Choice made a number of improvements to the drug tiering violations in the “Premier” and “Select” formularies, so that the majority of HIV treatment medications are no longer on the highest cost tiers. Unfortunately, their plans continue to discriminate against people living with HIV in other ways. “Select” plans, for instance, fail to cover any of the single-tablet regimens recommended as initial treatment for most people, exemplifying how Community Health Choice Texas plans fail to reflect treatment guidelines and clinical best practice. The plans also fail to comply with other important provisions of the ACA, including meeting the state Essential Health Benefits benchmark and providing no-cost coverage of PrEP, a USPSTF “A”-rated preventive service.
As a follow-up, in April, CCIIO met with people living with HIV, clinical providers, and others from Community Health Choice’s service area in southeast Texas, along with state Representative Venton Jones and staff of the HIV+Hepatitis Policy Institute. Community members described vividly the widespread negative impact of these plans. They described how the plans were already notorious for poor coverage of widely prescribed treatment regimens when they removed Biktarvy from the formulary in early 2023. Biktarvy, which is only available as a single-tablet regimen, is the most widely prescribed HIV treatment in the United States with a 49 percent market share.[2] Non-coverage of Biktarvy required many people with HIV in southeast Texas to stop the HIV treatment medication which they were already taking and successfully suppressing the HIV virus.
Clinicians confirmed that they saw these negative impacts among their own patients and described how newer single-tablet regimens are the standard of care not only in the United States, but around the world since they improve adherence, have more easily tolerated side effects, and have caused treatment failure rates to plummet. At that meeting, we presented a thorough review of HIV treatment guidelines and compared them to Community Health Choice formularies. While CCIIO included pharmacists as part of the meeting, unfortunately we did not receive any feedback on that analysis.
Despite these actions over the course of a year, Community Health Choice’s substandard formularies continue to be offered on the Marketplace and people living with HIV are still being subjected to them.
Since CCIIO has not taken any action against Community Health Choice, we will describe in more detail the way these formularies fail to reflect broadly accepted treatment guidelines and clinical best practice.
Community Health Choice Formularies Fail to Reflect HIV Treatment Guidelines and Clinical Best Practice
According to CFR 156.122 (a)(3)(iii)(H), insurance coverage must “[p]rovide[] appropriate access to drugs that are included in broadly accepted treatment guidelines and that are indicative of general best practices at the time.” [3] CCIIO’s written response to our complaint states that “CMS conducts several formulary reviews as part of the qualified health plan (QHP) certification process. The Non-Discrimination Clinical Appropriateness Review ensures that insurers offer sufficient numbers and types of drugs to effectively treat certain medical conditions, including HIV.” After comparing Community Health Choice Texas antiretroviral formularies with the United States Department of Health and Human Services Guidelines for the Use of Antiretroviral Agents for the Use of Antiretroviral Agents, we find it impossible to conclude that the insurer is in compliance with the regulation and these reviews were adequately completed.[4]
National treatment guidelines specify four types of treatment regimens for use as initial regimens by most people with HIV, as well as a fifth for use by individuals who have used cabotegravir as PrEP (Apretude) but for whom the results of resistance testing is not yet available. These regimens have “demonstrated durable virologic efficacy, favorable tolerability and toxicity profiles, and ease of use.”[5] Treatment guidelines go into detail about the many considerations involved in selecting a regimen for a person living with HIV. Coverage of these first-line regimens in Community Health Choice Premier and Select plans is summarized in Table 1 below.
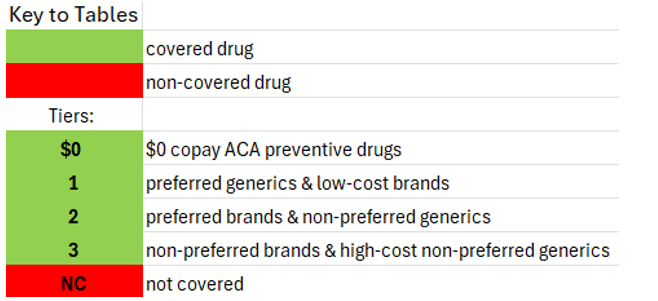
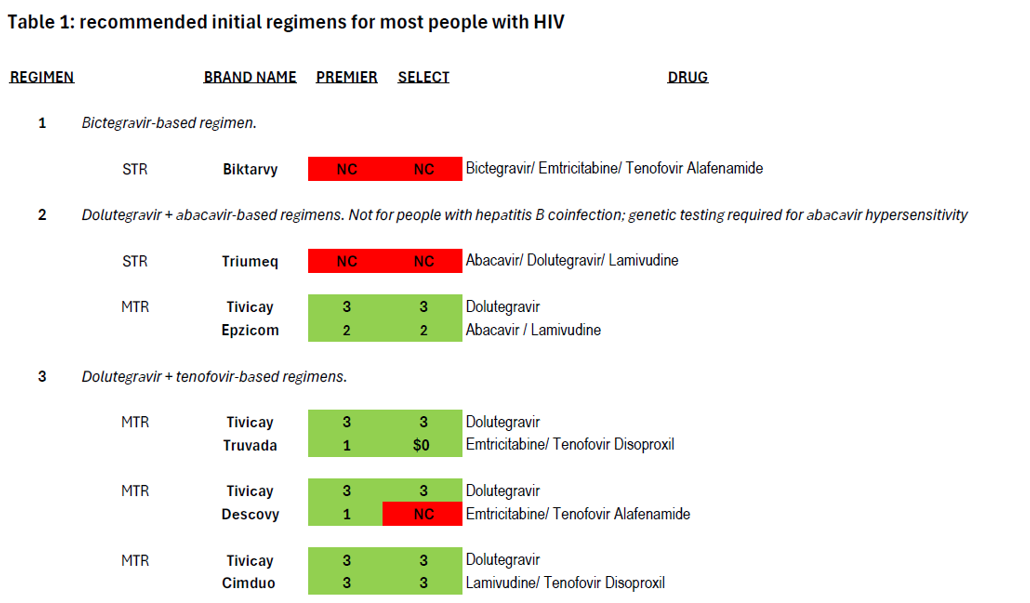

The treatment guidelines also specify nine additional regimen types which are “effective and tolerable but have some disadvantages” in comparison to the five regimen types recommended for most people.[6] These regimens are prescribed in certain clinical situations, such as high HIV viral load, HIV-associated dementia, and in the context of many comorbidities that impinge on HIV treatment such as viral hepatitis coinfection and other liver issues, cardiovascular disease, renal issues, bone issues, tuberculosis, or mental health.[7] Coverage of these nine regimens by Community Health Choice Premier and Select Plans is summarized in Table 2 below.
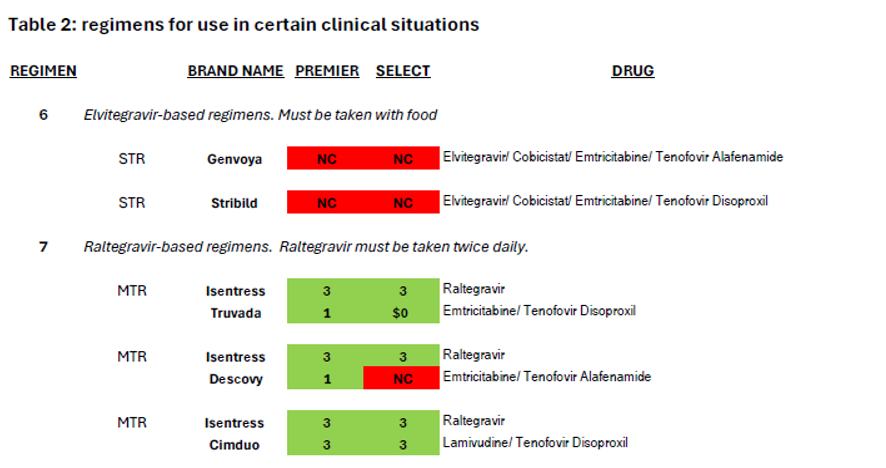
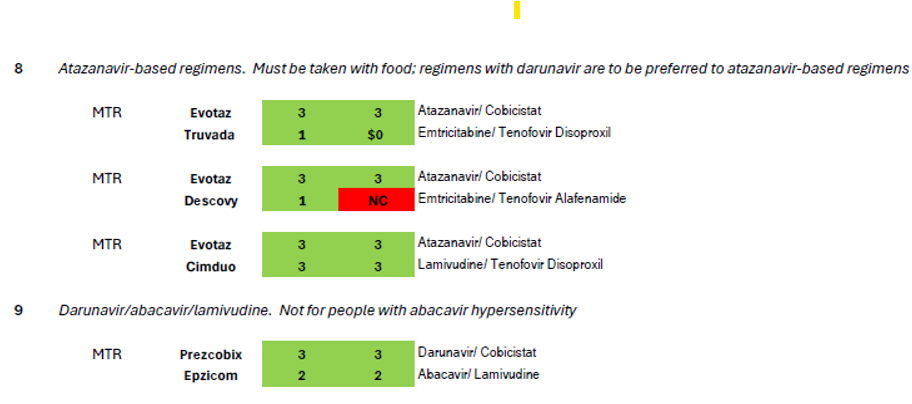
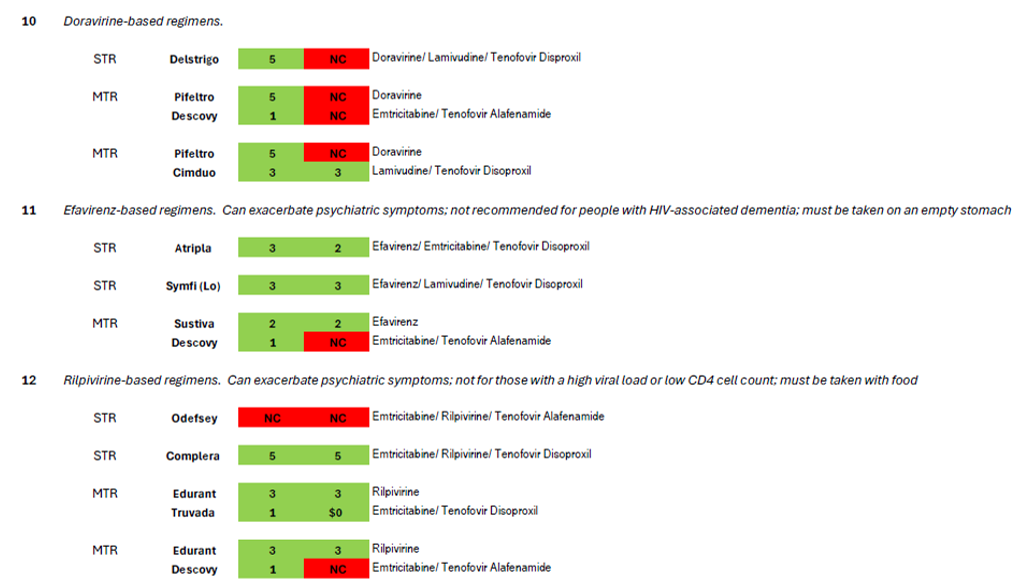

As illustrated by the number of drugs that are depicted in red blocks in these two tables, both these plans fall far short of meeting the HIV treatment guidelines for both initial treatment and for certain clinical circumstances.
We would like to highlight two egregious ways the number and type of drugs offered on Community Health Choice formularies fail to reflect treatment guidelines and broadly accepted clinical best practice:
- Coverage of single-tablet regimens (STRs)
- Coverage of regimens containing tenofovir alafenamide
Coverage of Single-Tablet Regimens
The availability of HIV treatment as STRs has revolutionized HIV treatment and improved medication adherence, allowing people living with HIV to take a single pill once daily rather than multiple pills with potentially different dosing schedules and requirements, such as to be taken with food or on an empty stomach.[8] As we have noted previously, CMS has specifically cited lack of coverage of single-tablet regimens for the treatment of HIV as potentially discriminatory.[9]
As we have mentioned, Biktarvy, the most commonly prescribed treatment regimen in the United States, is only available as a single-tablet regimen, and it is not covered by Community Health Choice Texas plans. Further, of the five regimens recommended for initial treatment for most people (Table 1), four are available as a single-tablet regimen (Biktarvy, Triumeq, Dovato, and Symtuza), but Community Health Choice Select plans do not cover any of these single-tablet regimens, meaning that enrollees in Select plans do not have any coverage for first-line regimens of a type that a majority of people on HIV treatment in the United States rely on. Three of these four regimens are available to Select plan enrollees but only when split up into a multi-tablet regimen, and a fourth is not available in a single-tablet formulation. These are clear violations of the ACA and severely limit treatment access to people living with HIV.
Turning to the nine additional regimens for use in certain clinical circumstances (Table 2), Community Health Choice plans fail to cover the elvitegravir-based regimens that are only available as STRs (#6 in Table 2, Genvoya or Stribild). Five single-tablet regimens are commercially available for three of the remaining eight regimen types: Delstrigo for #10; Atripla and Symfi (Lo) for #11, and Odefsey and Complera for #12. Of these five STRs, Select plans cover only Atripla, Symfi (Lo) and Complera, the first two of which are older efavirenz-based medications notorious for mental health side effects including suicidal ideation; the third is a medication using rilpivirine, which is also associated with the exacerbation of psychiatric side effects though not to the same extent. Given the higher prevalence of depression and other mental health comorbidities among people living with HIV compared to the general population,[10] it is perverse only to allow enrollees of Select plans to avail themselves of the single-tablet regimens with the highest risk of psychiatric exacerbation. Again, treatment guidelines are not being followed.
Coverage of Regimens Containing Tenofovir Alafenamide
Tenofovir is an antiretroviral that is frequently used as a part of a two-drug nucleoside reverse transcriptase inhibitor (NRTI) “backbone” of a three-drug treatment regimen. Of the fifteen treatment regimens enumerated in national recommendations for initial treatment (Table 1) and for use in certain clinical circumstances (Table 2), tenofovir is used in ten. Tenofovir comes in two forms: tenofovir disoproxil and tenofovir alafenamide. Tenofovir disoproxil, the older drug, is often associated with adverse effects on renal function and bone density, which means that the majority of people living with HIV have transitioned to regimens using tenofovir alafenamide, which has far less frequent adverse effects on individuals with renal or bone issues.[11]
Even though HIV treatment guidelines suggest weighing the greater bone and kidney toxicities of tenofovir disoproxil compared to tenofovir alafenamide as one of several key considerations when choosing an initial treatment regimen, Community Health Choice Select Plans do not cover any regimens (whether single- or multi-tablet) containing tenofovir alafenamide. The risk of tenofovir-associated adverse renal or bone side effects is even higher with regimens requiring a “booster” such as cobicistat, as with the darunavir- (Table 1, #5) elvitegravir- (Table 2, #6), and atazanavir-based (Table 2, #8) regimens, but here too, Select plans only allow for use of tenofovir disoproxil, offering no options for patients for whom renal and bone issues are a key concern. With a median age of 51,[12] people living with HIV in the United States increasingly need access to regimens that with less bone and renal toxicity.
In your December 2023 letter, you state that “we agree that Biktarvy, Dovato, Triumeq, and a combination drug therapy of dolutegravir + emtricitabine or lamivudine + tenofovir disoproxil or tenofovir alafenamide are recommended regimens indicated for people starting HIV treatment. Clinical guidelines used to develop our formulary reviews recommend both single tablet and multiple tablet regimens to manage the treatment of HIV, and issuers have the flexibility to determine which drugs to include on their formularies as determined by clinical evidence.” We wonder what clinical evidence can have guided Community Health Choice Texas to systematically exclude first-line single-tablet regimens (including Biktarvy, Dovato, and Triumeq) as well as regimens containing tenofovir alafenamide from their Select formulary.
Community Health Choice’s Deficient Formularies Also Fail to Comply with Other ACA Coverage Requirements
Community Health Choice plans also fail to comply with other important ACA coverage protections:
- Community Health Choice plans do not meet Texas Essential Health Benefits benchmark plan standards for the coverage of antiretrovirals:
- Select Formularies do not appear to meet the Texas EHB benchmark for the Nucleoside Reverse Transcriptase Inhibitor class, which requires coverage of eleven drugs listed in the NRTI category in the United States Pharmacopeia Medicare Model Guidelines version 9.0.[13]
- Premier and Select formularies do not appear to meet the Texas EHB benchmark for the “other” class of antiretrovirals, which requires the coverage of three drugs listed in this category in the United States Pharmacopeia Medicare Model Guidelines version 9.0.[14]
- The plans appear to fall short of EHB benchmarks despite padding the formularies by covering old, rare, or discontinued antiretrovirals:
- Select:
- NRTIs: Trizivir (abacavir/lamivudine/zidovudine) and Retrovir (zidovudine)
- protease inhibitors: Crixivan (indinavir)
- Premier:
- NRTIs: Trizivir (abacavir/lamivudine/zidovudine); Retrovir (zidovudine); Videx (didanosine); Zerit (stavudine)
- Protease inhibitors: Crixivan (indinavir); Invirase (saquinavir)
- NNRTIs: Rescriptor (delavirdine)
- Premier formularies do not list a PrEP medication that is covered in the $0 preventive tier. The only PrEP drug covered is Truvada or its generic equivalent, listed on Tier 1 on Premier formularies.
We would note that HHS guidelines have just been updated in September, with four of the nine regimens specified for use in certain clinical situations removed from the recommendations citing “higher pill burdens, more adverse effects, or a lower barrier to resistance than other ART regimens”. This would put Community Health Choice Texas formularies more out of line with current treatment guidelines and we urge CCIIO to ensure all plans nationwide are in compliance with the updated guidelines.[15]
We urge CMS once again to take immediate action to compel Community Health Choice Texas plans to bring coverage in line with HIV treatment guidelines and into compliance with all ACA coverage requirements. As we noted in our September 2023 letter, a complaint was filed against Community Health Choice Texas for adverse tiering of HIV treatment medications in 2016, though it is unclear if any enforcement actions took place at that time.
Your letter of December 2023 states that “we also identified that these plans cover at least seven single tablet regimens, including Atripla, Complera, Delstrigo, Dovato, Juluca, Symfi, and Trizivir.” This statement was then and remains true today only of Premier formularies. Select formularies cover only Atripla, Complera, Symfi, and Trizivir (with this last a very old and rarely used STR containing AZT, the first antiretroviral drug approved in 1987 with a notorious side effect burden).
We look forward to learning what actions you have taken or will take in regards to Community Health Choice Texas and to ensure that all 2025 Marketplace plans meet ACA requirements for non-discriminatory coverage of HIV treatment.
If you have any questions, comments, or would like to discuss these issues further, please contact Carl Schmid, Executive Director, HIV+Hepatitis Policy Institute at cschmid@hivhep.org or (202) 462-3042, or Kevin Herwig, Health Policy Manager, HIV+Hepatitis Policy Institute at kherwig@hivhep.org or (617) 666-6634.
Sincerely,

Carl E. Schmid, II
Executive Director
cc: Jeff Wu, Deputy Director for Policy, CCIIO
Melanie Fontes Raine, Director, HHS Office for Civil Rights
Cassandra J. Brown, Texas Commissioner of Insurance
Dr. Laura Cheever, Associate Administrator, HIV/AIDS Bureau, HRSA
Francisco Ruiz, Director, White House Office of National AIDS Policy
Marlene McNeese, Co-Chair, Presidential Advisory Commission on HIV/AIDS and Deputy Assistant Director, Houston Health Department
[1] https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-B/part-156/subpart-B/section-156.122
[2] https://s29.q4cdn.com/585078350/files/doc_financials/2024/q2/GILD-Q224-Earnings-Presentation-8-August-2024.pdf, slide 10
[3] https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-B/part-156/subpart-B/section-156.122
[4] https://clinicalinfo.hiv.gov/en/guidelines
[5] For the purposes of this letter, we will describe HHS guidelines in effect from September 2022 until a recent significant update in September 2024. All five regimens described here were recommended for use as initial treatment since September 2022 until one regimen was moved to the section on regimens “for use in certain clinical situations” in September 2024): https://clinicalinfo.hiv.gov/sites/default/files/guidelines/archive/adult-adolescent-arv-2022-09-21.pdf (Table 6a)
[6] A tenth, darunavir and tenofovir-based regimen is enumerated both as a recommended initial therapy for persons with a history of use of cabotegravir as PrEP who have not had required genetic resistance testing as well as for use under certain clinical circumstances.
[7] For the purposes of this letter, we will describe HHS guidelines in effect from September 2022 until a recent significant update in September 2024. The nine regimens described here were all recommended for use in certain clinical recommendations since June 2021 until four of them were removed from the list in September 2024): https://clinicalinfo.hiv.gov/sites/default/files/guidelines/archive/AdultARV_GL_2021_2021_06_03.pdf (Table 6b)
[8] Hemmige, V., Flash, C. A., Carter, J., Giordano, T. P., & Zerai, T. (2018). Single tablet HIV regimens facilitate virologic suppression and retention in care among treatment naïve patients. AIDS Care, 30(8), 1017–1024. https://doi.org/10.1080/09540121.2018.1442554 ; Kapadia, S. N., Grant, R. R., German, S. B., Singh, B., Davidow, A. L., Swaminathan, S., & Hodder, S. (2018). HIV virologic response better with single-tablet once daily regimens compared to multiple-tablet daily regimens. SAGE Open Medicine, 6, 205031211881691. https://doi.org/10.1177/2050312118816919
[9] See 2017 Letter to Issuers, page 45: https://www.cms.gov/cciio/resources/regulations-and-guidance/downloads/final-2017-letter-to-issuers-2-29-16.pdf
[10] Angelino AF et al 2001, Clinical Infectious Diseases 33 847 https://academic.oup.com/cid/article/33/6/847/330176
[11] Baumgardner J et al 2018, AJMC 34 SP322 “Modeling the Impacts of Restrictive Formularies on Patients with HIV,” https://ajmc.s3.amazonaws.com/_media/_pdf/AJMC_07_2018_PharmacyBenefits_SpecialIssue_Baumgardner%20final.pdf
[12] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8795488/
[13] The ten NRTI drugs covered by Select formularies are: abacavir (Ziagen), emtricitabine (Emtriva), lamivudine (Epivir), tenofovir disoproxil (Viread), zidovudine (Retrovir) and the combination drugs Cimduo, Combivir, Epzicom, Trizivir, and Truvada
[14] The two drugs in the “other” class covered by Premier and Select formularies are enfuvirtide (Fuzeon) and maraviroc (Selzentry)
[15] https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/whats-new