Substandard & discriminatory HIV medication plan design and coverage by Medica in Iowa

Commissioner
Iowa Insurance Division
1963 Bell Avenue, Suite 100
Des Moines IA 50315
Dear Commissioner Ommen:
The HIV+Hepatitis Policy Institute is a national, non-profit organization whose mission is to promote quality and affordable healthcare for people living with or at risk of HIV, hepatitis, and other serious and chronic health conditions.
We are writing to express our concern about substandard, discriminatory coverage of HIV treatment medications by Medica health plans in Iowa.[1]
- Medica’s plans engage in a practice known as “adverse tiering,” in which all or the majority of drugs to treat a particular health condition are placed on the highest cost-sharing tiers.
- Medica’s plans fail to cover treatment regimens recommended in broadly accepted treatment guidelines and that are indicative of clinical best practice.
- Medica’s plans fail to meet Iowa’s Essential Health Benefits (EHB) Benchmark Plan standards for coverage of antiretrovirals.
These benefit designs, which discourage enrollment by Iowans living with HIV, are plainly discriminatory. We urge the Iowa Insurance Division, which reviews, approves, and regulates Marketplace plans in Iowa, to take immediate action against Medica for offering these substandard plans that violate the ACA and its implementing regulations. We urge you to ensure that these violations are rectified before the new plan year begins.
Medica’s Plans Use Adverse Tiering to Discourage Enrollment by People Living with HIV
Adverse tiering occurs when an issuer places all or a majority of drugs to treat a particular health condition on a high-cost formulary tier.[2] According to the 2023 Notice of Benefit and Payment Parameters, formularies that employ adverse tiering discourage enrollment by people living with HIV, and are presumptively discriminatory.
Medica’s formulary uses five tiers:
- Tier 1. ACA preventive drugs
- Tier 2. Preferred Generics
- Tier 3. Preferred Brands, Non-Preferred Generics
- Tier 4. Non-Preferred Brands, Non-Preferred Generics
- Tier 5. Specialty Prescription Drugs
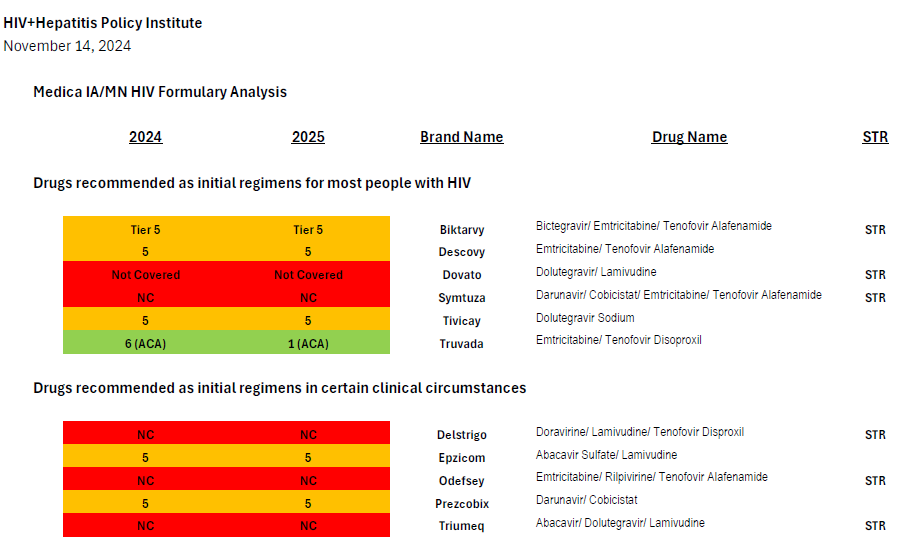
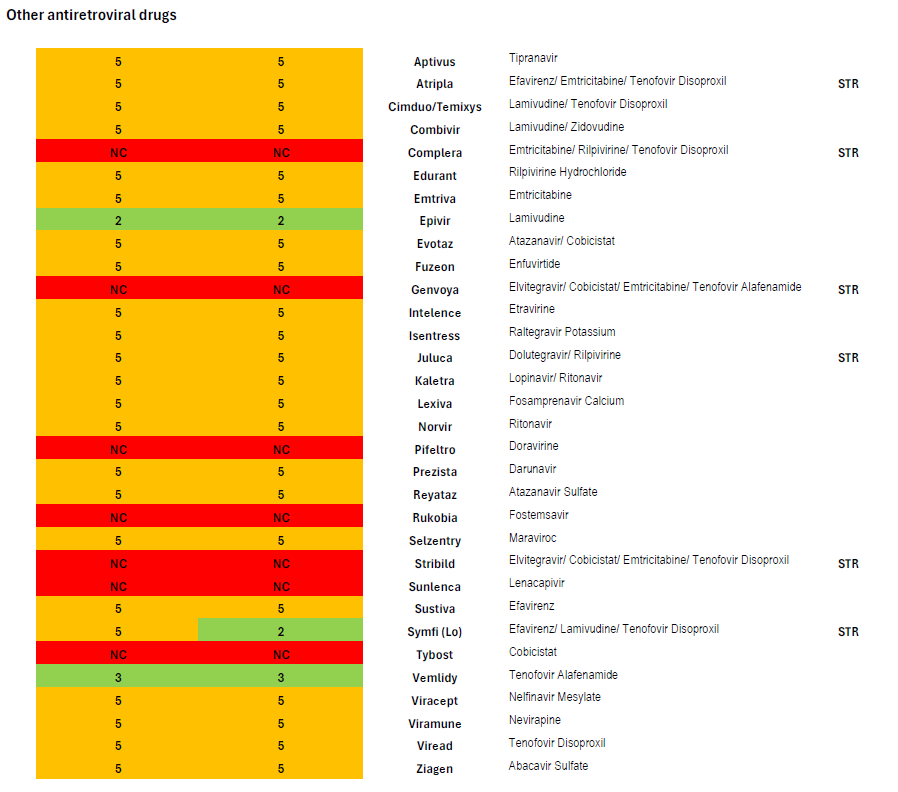
Medica’s coverage of antiretrovirals through the prescription drug benefit in the 2024 and 2025 plan years is summarized in the attached chart, in which drugs covered in Tier 5 appear in orange.
In 2024, Medica placed every single HIV treatment drug but two (lamivudine/Epivir and tenofovir alafenamide/Vemlidy) on its highest cost specialty tier, Tier 5.[3] This even includes very low cost generics and is a clear case of adverse tiering taken to an extreme.
Lamivudine and tenofovir alafenamide are both also used as a treatment for hepatitis B, and neither drug can be used as HIV treatment by itself, only in combination with one or two other drugs—so every single enrollee living with HIV in Medica’s plans in 2024 was required to use medications from the highest-cost specialty tier. The fact that the only antiretrovirals that are not adversely tiered are used as treatments for hepatitis B also suggests that the intent of Medica’s formulary design is to discourage enrollment by people with HIV.
Medica’s 2025 plans, which were recently released, have modified this discriminatory tiering slightly: a generic single-tablet regimen (efavirenz/lamivudine/tenofovir disoproxil or Symfi) is now covered on the lowest-cost tier. However, efavirenz-based regimens are increasingly rarely prescribed due to their neuropsychiatric side effect burden.
Specialty tier drugs are associated with high out-of-pocket costs. For example, enrollees in IA Inspire by Medica Expanded Bronze Standard must pay a $500 copay after a deductible of $7,500 for individuals and $15,000 for families. IA Inspire by Medica Bronze $0 Copay PCP Visits requires a $750 copay after a deductible of $7,500 for individuals and $15,000 for families. IA Inspire by Medica Bronze Share requires a monthly copay of $850. This means a Medica plan enrollee living with HIV can face out-of-pocket costs higher than the list price of a low-cost generic.
These high out-of-pocket costs will also harm Iowa’s AIDS Drug Assistance Program (ADAP), whose Insurance Assistance Program pays out-of-pocket costs for their enrollees, and force ADAP to pay more for their clients.
Medica’s Plans Fail to Reflect HIV Treatment Guidelines and Clinical Best Practice
Medica’s coverage of antiretrovirals also fails to cover drugs that are recommended in broadly accepted treatment guidelines and that are indicative of general best practices in the United States. This also discourages enrollment by people living with HIV and violates regulatory standards for formulary adequacy.[4]
After comparing Medica’s coverage of antiretroviral drugs with the United States Department of Health and Human Services Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV, it is clear that the insurer is not in compliance with the regulation and clinically-based reviews were not adequately completed. Coverage of antiretrovirals is summarized in the chart attached.
Coverage of Preferred Initial Regimens Recommended as Initial Therapy for Most People
Current guidelines recommend four preferred regimens as initial therapy for most people (Biktarvy, Dovato, Symtuza, or a combination of Tivicay with either Truvada or Descovy).[5] Medica’s plans only cover two preferred regimens: Biktarvy or Tivicay + Truvada/Descovy.[6] The recommended regimens are preferred due to their “demonstrated durable virologic efficacy, favorable tolerability and toxicity profiles, and ease of use.”
Symtuza is the only recommended initial regimen for individuals who have taken the new long-acting injectable PrEP medication Apretude (cabotegravir) but are starting antiretroviral therapy before results of resistance testing are available. Dovato is the only two-drug regimen recommended as initial therapy for most people. Covering only two (Biktarvy and Tivicay + Truvada/Descovy) out of four preferred initial regimens is not sufficient coverage.
Coverage of Alternative Initial Regimens Recommended as Initial Therapy In Certain Clinical Circumstances
Current guidelines recommend four alternative regimens as initial therapy in certain clinical circumstances (Delstrigo, Odefsey, Triumeq, and a combination of Prezcobix and Epzicom). Of these four alternative regimens, only one (Prezcobix + Epzicom) is covered—the only one of the four regimens which is not available as a single-tablet regimen (STR).
The eight preferred and alternative regimens described above are only the most widely applicable set of recommendations in national treatment guidelines, but Medica fails to cover half of these critically important and broadly prescribed regimens.
Coverage of Single-Tablet Regimens
The availability of HIV treatment as STRs has revolutionized HIV treatment and improved medication adherence, allowing people living with HIV to take a single pill once daily rather than multiple pills with potentially different dosing schedules and requirements, such as to be taken with food or on an empty stomach.[7] Six of the eight preferred and alternative regimens recommended in national treatment guidelines as initial therapy are available as STRs. National treatment guidelines, last updated in September, cite availability as once-daily therapy and pill burden as the reason some previously recommended regimens, are no longer recommended.[8] The broad uptake of STRs, which are prescribed to a large majority of people on HIV treatment in the United States, show that the choice of an STR is indicative of clinical best practice.
Thirteen STRs are available, of which Medica only covers four. As described above, of the three STRs recommended as one of four preferred regimens (Biktarvy, Dovato, and Symtuza), only one (Biktarvy) is covered; of the three STRs recommended as one of four alternative regimens in certain clinical circumstances (Delstrigo, Odefsey and Triumeq), none are covered.
The pattern of non-coverage of STRs continues among other antiretroviral medications: Complera, Genvoya, and Stribild, which are all widely prescribed and which were among the alternative regimens recommended in certain circumstances until September 2024, are also not covered. Other than Biktarvy, Medica also covers Juluca, Atripla, and Symfi. Atripla and Symfi are older efavirenz-based medications notorious for mental health side effects including suicidal ideation.
It is clear that this formulary, with its lack of STR coverage, is not based on clinical evidence. CMS has specifically cited lack of coverage of single-tablet regimens for the treatment of HIV as potentially discriminatory.[9]
Medica’s Plans Do Not Meet Iowa’s Essential Health Benefits Benchmark for Coverage of Antiretrovirals
Iowa, like all states, has selected an EHB benchmark plan which defines the minimum number of drugs plans must cover in each category and class.[10] Medica plans do not meet Iowa’s Essential Health Benefits benchmark plan standards for the coverage of antiretrovirals in two of the five classes in the antiretroviral category in the current version of the United States Pharmacopeia Medicare Model Guidelines (USP MMG).
Iowa’s current benchmark plan requires coverage of thirteen drugs listed in the “nucleoside/nucleotide reverse transcriptase inhibitor (NRTI)” class of antiretrovirals in the current version of the United States Pharmacopeia Medicare Model Guidelines (version 9.0), but these formularies cover twelve.[11] However, of the twelve medications classed as NRTIs Medica covers, three—zidovudine, didanosine, and stavudine—are old, rarely prescribed drugs from the earliest days of antiretroviral drug development. Medica could instead have opted to cover widely prescribed and guideline-recommended medications such as Odefsey or Triumeq, which are included in the NRTI class in the USP MMG.
Iowa’s benchmark plan also requires the coverage of at least three drugs in the “other” class of antiretroviral drugs. Of the five medications listed in this category in the USP MMG, Medica covers only two, maraviroc (Selzentry) and Fuzeon (enfuvirtide). Fuzeon will be discontinued on February 28, 2025, and no generic is available.[12] An important member of the USP MMG “other” class that Medica does not cover is Sunlenca (lenacapavir), a new first-in-class long-acting antiretroviral recommended by national guidelines and an option for the treatment of heavily treatment-experienced people living with HIV who often have developed multiple resistance mutations that leave the patient unable to take certain drugs or classes of drugs. [13]
We urge the Iowa Insurance Division to take immediate action to bring Medica tiering and coverage in line with all ACA coverage requirements and HIV treatment guidelines.
Failure to address benefit designs that discriminate against people with HIV has grave consequences for individual and public health. When preferred drugs are not covered people will likely discontinue treatment and jeopardize their own health and lead to new transmissions of HIV. Failure to enforce ACA protections against discriminatory benefit designs will inevitably worsen health inequities. Black and Latino communities in Iowa are disproportionately affected by HIV. Black people make up 3.7 percent of Iowa’s population but comprise 24.4 percent of new diagnoses; Latino people are 6.5 percent of the population but make up 20.3 percent of new diagnoses. [14]
We look forward to learning what actions you will take regarding Medica’s formulary in order to ensure that all 2025 Marketplace and other state-regulated plans in Iowa meet ACA requirements for non-discriminatory coverage of HIV treatment.
If you have any questions, comments, or would like to discuss these issues further, please contact Carl Schmid, Executive Director, HIV+Hepatitis Policy Institute at cschmid@hivhep.org or (202) 462-3042, or Kevin Herwig, Health Policy Manager, HIV+Hepatitis Policy Institute at kherwig@hivhep.org or (617) 666-6634.
Sincerely,

Carl E. Schmid, II
Executive Director

cc: Ellen Montz, Director, CCIIO
Jeff Wu, Deputy Director for Policy, CCIIO
Melanie Fontes Raine, Director, HHS Office for Civil Rights
Dr. Laura Cheever, Associate Administrator, HIV/AIDS Bureau, HRSA
Francisco Ruiz, Director, White House Office of National AIDS Policy
[1] We are submitting a similar complaint to insurance regulators in Minnesota, where Medica offers an identical formulary.
[2] “Using Drugs to Discriminate—Adverse Selection in the Insurance Marketplace,” Douglas B. Jacobs and Benjamin D. Sommers, New England Journal of Medicine, January 28, 2015, DOI: 10.1056/NEJMp1411376.
[3] The plans also put emtricitabine/tenofovir disoproxil/Truvada correctly in an ACA preventive tier due to its use as PrEP.
[4] https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-B/part-156/subpart-B/section-156.122
[5] https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/tables-adult-adolescent-arv.pdf (Table 6a)
[6] Coverage of Cimduo can also substitute for coverage of Truvada.
[7] Hemmige, V., Flash, C. A., Carter, J., Giordano, T. P., & Zerai, T. (2018). Single tablet HIV regimens facilitate virologic suppression and retention in care among treatment naïve patients. AIDS Care, 30(8), 1017–1024. https://doi.org/10.1080/09540121.2018.1442554 ; Kapadia, S. N., Grant, R. R., German, S. B., Singh, B., Davidow, A. L., Swaminathan, S., & Hodder, S. (2018). HIV virologic response better with single-tablet once daily regimens compared to multiple-tablet daily regimens. SAGE Open Medicine, 6, 205031211881691. https://doi.org/10.1177/2050312118816919
[8] https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/whats-new
[9] See 2017 Letter to Issuers, page 45: https://www.cms.gov/cciio/resources/regulations-and-guidance/downloads/final-2017-letter-to-issuers-2-29-16.pdf
[10] 45 CFR 156.122 https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-B/part-156/subpart-B/section-156.122
[11] Descovy, Truvada, Epzicom, Cimduo, Combivir, Emtriva, Epivir, Viread, Ziagen, Retrovir, Videx, Zerit
[12] https://www.gene.com/media/statements/ps_081924
[13] We do not know if Sunlenca is covered through the medical benefit.
[14] https://map.aidsvu.org/profiles/state/iowa/overview (accessed on November 13, 2024)